Introduction
Materials and Methods
Plant Materials
cDNA Synthesis and Extraction of DNA and RNA
Linkage and Marker Analysis
Cloning and Sequence Analysis of PSY1
Carotenoid Extraction and High-Performance Liquid Chromatography (HPLC) Analysis
Expression Analysis of the Carotenoid Biosynthesis Genes
Results
Carotenoid Analysis of YF2359 by HPLC
Linkage Analysis of the Yellow Fruit in YF2359
Analysis of the PSY1 Transcripts in YF2359
Expression Analysis of the Carotenoid Biosynthesis Genes in YF2359
Discussion
Introduction
Tomato (Solanum lycopersicum) is an important crop that provides various nutrients, including vitamins, minerals, and carotenoids (Guil-Guerrero and Rebolloso-Fuentes, 2009). It is also the most studied model system for fleshy fruit biology. In tomato, fruit color is a crucial quality trait that affects consumer preferences. During fruit ripening, the chlorophyll-containing chloroplasts are converted to chromoplasts, which gradually accumulate more carotenoids. Consequently, fruit color successively changes from green to yellow, orange, or red (Egea et al., 2010).
Carotenoids are isoprenoids with critical roles in photoprotection and light absorption (Enfissi et al., 2017). In addition, carotenoids are the precursors of various plant hormones, such as abscisic acid, and represent one of the largest classes of natural pigments, conferring the bright-red, orange, and yellow hues of fruits, flowers, and seeds (Dorais et al., 2008; Ahrazem et al., 2016). Carotenoids provide various human health benefits, including prevention of chronic diseases, cancers, and visual impairment. Humans cannot synthesize carotenoids de novo and depend exclusively on the consumption of carotenoid-containing foods for these nutrients (Fraser and Bramley, 2004).
Carotenoids are synthesized by the methylerythritol 4-phosphate pathway. The isopentenyl diphosphate (Shinozaki et al., 2018) and dimethylallyl diphosphate produced in the methylerythritol 4-phosphate pathway undergo a condensation reaction to form the phytoene precursor geranylgeranyl diphosphate (GGPP). Two GGPP molecules are then condensed by phytoene synthase (PSY) to produce phytoene, which is further converted to phytofluene by phytoene desaturase. Subsequently, ζ-carotene isomerase and ζ-carotene desaturase consecutively synthesize cis-lycopene from phytofluene. Carotenoid isomerase then converts cis-lycopene to trans-lycopene. The cyclization of lycopene by lycopene cyclases is a critical step in directing the metabolic flux of the bifurcated carotenoid pathway. Both open ends of lycopene can be cyclized. Cyclization by lycopene ε-cyclase or β-cyclase (LCYB) generates ε- or β-ionone rings, respectively. The combination of β- and ε-ionone rings produces δ-carotene and α-carotene and its derivate lutein. Conversely, when two β-ionone rings are formed, β-carotene and its derivatives, for example, neoxanthin and zeaxanthin, are produced (Cunningham, 2002; Fraser and Bramley, 2004). γ-Carotene has one β-ring and one uncyclized end, and δ-carotene has one ε-ring and one uncyclized end. The accumulation of trans-lycopene increases during fruit ripening as a result of reduced lycopene cyclization and up-regulation of PSY1 (Fraser et al., 2002).
Characterization of several mutants in tomato has contributed to the understanding of carotenoid biosynthesis and metabolism. For example, the yellow flesh (r) mutant has ripe yellow fruit because of a loss-of-function mutation in PSY1, leading to a lack of carotenoids during fruit ripening (Fray and Grierson, 1993). The tangerine (t) mutant has ripe orange fruit resulting from the accumulation of prolycopene instead of trans-lycopene because of the deletion of carotenoid isomerase (Isaacson et al., 2002). The Beta (B) mutant also has ripe orange fruit, but this phenotype is caused by a high accumulation of β-carotene because of LCYB over-expression (Ronen et al., 2000). The Delta mutant, which shows lycopene-ε-cyclase up-regulation, produces ripe orange fruit as a result of increased accumulation of δ-carotene and decreased accumulation of the substrate trans-lycopene (Ronen et al., 1999; Yoo et al., 2017). Additionally, mutation in genes that control the ripening process influence the fruit color. For example, the ripening inhibitor (rin) mutant produces yellow fruit that do not ripen because of the deletion of the intergenic region between LeMADS-RIN and LeMADS-MC, and part of LeMADS-RIN (Vrebalov et al., 2002). The Colorless non-ripening (Cnr) mutant also produces yellow fruit, but this phenotype results from the methylation of the promoter of the SBP-box (SQUAMOSA promoter binding protein-like) gene (Manning et al., 2006).
PSY is the most studied gene family in the carotenoid biosynthesis pathway. To date, three PSY homologs have been identified in tomato. Among them, PSY1 is highly expressed during fruit ripening, whereas PSY2 mainly functions in vegetative tissues. PSY3 is reported to regulate carotenoid biosynthesis in the root (Kang et al., 2014; Liu et al., 2015). During fruit ripening, the transcript level and enzyme activity of PSY1 are increased (Ronen et al., 1999). In addition, PSY1 has a higher flux control coefficient, which is a quantitative indicator of the influence of an enzyme on carotenoid biosynthesis. Therefore, PSY1 is the critical enzyme involved in the first committed step of the carotenoid biosynthesis pathway in fruit (Fraser et al., 2002). Various mutations related to PSY1 have been reported. For example, a transcriptional lesion resulting from a mutation of Rider insertion in PSY1 results in the r mutant (Cheng et al., 2009). The ry mutant results from a small truncation of the 3ʹ end of PSY1 (Jiang et al., 2012). Sugar Yellow tomato has a single T/A substitution in the sixth exon of PSY1 that results in a premature stop codon (Shin et al., 2019). Galina, lidi, and T1039 produce yellow-colored cherry tomato fruit because of a single G deletion in PSY1. This deletion results in an amino acid substitution from Lys389 to Ser, leading to a premature stop codon (Aflitos et al., 2014). Here, we identified two PSY1 transcripts in the yellow-fruited tomato YF2359 by linkage and sequence analysis. Both PSY1 transcripts were predicted to be generated by trans-splicing. Information on this unusual mutation in PSY1 caused by trans-splicing will elucidate a wide spectrum of genetic variations in plants and can be used to improve the fruit quality of tomato.
Materials and Methods
Plant Materials
Solanum lycopersicum ‘M82’, S. lycopersicum ‘E6203’, and S. pimpinellifolium ‘LA1589’ show ripe red fruit. Solanum lycopersicum ‘LA4044’, known as yellow flesh (r), shows ripe yellow fruit. Only cultivar names are used hereafter. Seeds were obtained from the Tomato Genetic Resource Center (University of California, Davis, CA, USA). Seeds of S. lycopersicum ‘YF2359’ were obtained from Nongwoo Bio, Korea (Fig. 1A). YF2359 was crossed with LA1589 to produce F2 populations. All the plants were grown in the greenhouse and field at Kyungpook National University, Daegu, Korea. The pericarps of ripe fruits were collected, immediately frozen in liquid nitrogen, and ground for extraction of carotenoids and total RNA.
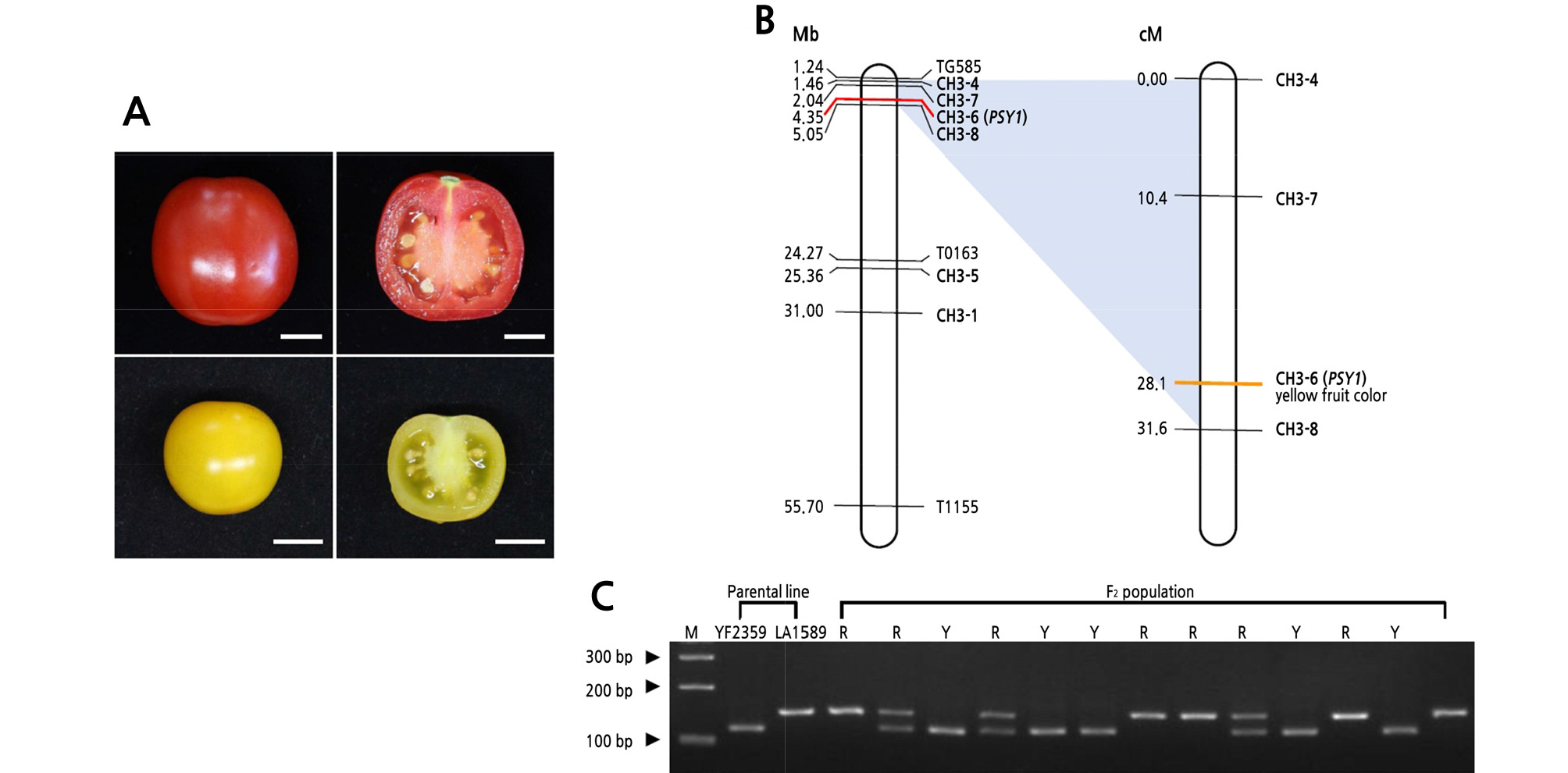
Fig. 1.
Linkage analysis of yellow fruit color. (A) Ripe fruit of Solanum lycopersicum ‘M82’ (top) and YF2359 (bottom). Ripe fruit was harvested 10 days after the breaker stage. The scale bars indicate 1 cm. (B) Physical (left) and linkage maps (right) for genetic mapping of the yellow fruit color on chromosome 3. A total of 235 F2 individuals (YF2359 × LA1589) were genotyped with five InDel markers and one dCAPS marker (CH3-6 marker). Two markers (CH3-7 and CH3-8) were described previously (Liu et al., 2017). The yellow fruit color was mapped to a 28.1-cM region with PSY1. (C) Co-segregation analysis of yellow fruit color and PSY1. The dCAPS marker was based on a substitution in the promoter region of PSY1 in LA1589. The PCR products amplified by CH3-6 marker (PSY1) were digested with Mnl I and separated on 3% agarose gels. Numbers on the left indicate the DNA ladder size; “M” indicates the 1-kb DNA ladder; “R” and “Y” indicate red and yellow fruit color in the F2 population, respectively.
cDNA Synthesis and Extraction of DNA and RNA
Genomic DNA was extracted from leaves using the cetyltrimethylammonium bromide method (Murray and Thompson, 1980). After total RNA was isolated using a RibospinTM Seed/Fruit Kit (GeneAll Biotechnology Co., Ltd., Seoul, Korea), cDNA was synthesized using a DiaStarTM RT Kit (SolGent Co., Ltd., Daejeon, Korea).
Linkage and Marker Analysis
A total of 235 F2 individuals (YF2359 × LA1589) were utilized to conduct a linkage analysis of the yellow fruit. The fruit color phenotype and genotype data of the F2 population were used to construct the linkage map using MAPMAKER/EXP 3.0 (Lander et al., 1987). Polymerase chain reaction (PCR) was performed using a T100TM Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) under the following conditions: 95°C for 3 min, followed by 35 cycles of 95°C for 30 s, annealing temperature for 30 s, 72°C for 1 min, and 72°C for 5 min. The reaction mixture was composed of 18.875 µL of ddH2O, 1 µL of DNA (200 ng), 0.5 µL of 10 mM dNTP mix, 2.5 µL of 10× e-Taq DNA reaction buffer, 1 µL of 10 pmol forward and reverse primer each, and 0.125 µL of SolgTM e-Taq DNA polymerase (SolGent Co., Ltd.).
Cloning and Sequence Analysis of PSY1
Isolation of total RNA from the ripe fruit pericarps of M82 and YF2359 and synthesis of cDNA were undertaken using commercial kits, as mentioned above. The PSY1 promoter and coding region in M82 and YF2359 were cloned with two and one primer set, respectively (Suppl. Table 1s.). PCR amplification was conducted using SolgTM e-Taq DNA polymerase (SolGent Co., Ltd.) according to the manufacturer’s instructions. Extension of the 3ʹ-untranslated region (3'-UTR) in YF2359 was carried out using classic rapid amplification of cDNA ends, as previously described (Scotto ‑ Lavino et al., 2006). All amplified PCR products were cloned using a T-bluntTM PCR Cloning Kit (SolGent Co., Ltd.) and the Escherichia coli strain DH5α. Plasmid DNA was isolated by an MG Plasmid DNA MiNi Kit (MGmed Co., Ltd., Seoul, Korea), and three clones per cultivar were sequenced at SolGent Co., Ltd. ClustalX 2.0 (https://clustalx.software. informer.com/2.1/) and GeneDoc (https://genedoc.software.informer.com/2.7/) programs were used for sequence alignment.
Carotenoid Extraction and High-Performance Liquid Chromatography (HPLC) Analysis
Approximately 0.1 g of frozen pericarp powder from ripe fruit was added to a 2-mL screw-cap tube containing two 6-mm glass beads for carotenoid extraction, as previously described (Yoo et al., 2017). Extracted carotenoids were analyzed using a 1260 Infinity HPLC (Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with a YMC Carotenoid C30 S-5 column (4.6 × 250 mm) and Agilent ChemStation software (Agilent Technologies, Inc.). A two-solvent mobile phase system consisting of MeOH with 0.1% ammonium acetate (solvent A) and methyl tert-butyl ether (solvent B) was used, with detection at 286, 348, 434, and 450 nm. Each carotenoid was identified by the absorption maxima spectrum (Gupta et al., 2015).
Expression Analysis of the Carotenoid Biosynthesis Genes
Total RNA of ripe fruit and the corresponding cDNA were obtained for quantitative real-time (qRT)-PCR using commercial kits by following the manufacturers' instructions, as described above. qRT-PCR was performed with 100 ng of the cDNA and gene-specific primers (Suppl. Table 1s.) using the Power SYBR® Green PCR Master Mix (Applied Biosystems, Waltham, MA, USA) according to the manufacturer’s instructions. At least three biological and two technical replicates were set up for analysis by qRT-PCR. Amplifications were conducted on a CFX ConnectTM Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) under the following conditions: 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min, followed by a melting curve of 95°C for 10 s, 60°C for 5 s, 95°C for 5 s, and 22°C for 5 s. ACTIN was used as an internal control for normalization.
Results
Carotenoid Analysis of YF2359 by HPLC
The HPLC carotenoid profiles of M82 (red fruit), E6203 (red fruit), and YF2359 (yellow fruit) revealed that YF2359 had a different carotenoid profile from M82 (Fig. 1A). Lutein, β-carotene, γ-carotene, and trans-lycopene were detected in YF2359, whereas phytoene, phytofluene, and cis-lycopene were not. Although the β- and γ-carotene levels in YF2359 were only 2-fold lower than those in M82, the trans-lycopene and lutein levels were 120- and 6-fold lower, respectively. The carotenoid levels in YF2359 were also found to be lower than those in E6203 (Table 1) but similar to those in LA4044, known as yellow flesh (r) mutant, as described previously (Shin et al., 2019). Phytofluene and cis-lycopene were not detected in LA4044. The total carotenoid level in YF2359 and LA4044 was 6.61 ± 2.10 and 8.54 ± 3.12 µg·g -1 fresh weight, respectively. Therefore, YF2359 was predicted to have a PSY1 mutation, leading to a lack of carotenoids in ripe fruit. The yellow fruit of the rin mutant is reported to have enhanced firmness (Vrebalov et al., 2002). To identify whether the YF2359 fruit had the yellow flesh phenotype because of a ripening defect, we compared YF2359 fruit firmness with that of M82 tomato fruit. However, there was no significant difference in the firmness between YF2359 and M82 (Suppl. Fig. 1s.). From the combined fruit firmness data and carotenoid profiles, YF2359 was predicted to have a defect of carotenoid accumulation rather than a defect of ripening. We hypothesized that there could be a PSY1 sequence variation in YF2359 and so we analyzed the sequences of the PSY1 promoter and coding regions in YF2359. No sequence variation was found (Suppl. Fig. 2s.); thus, we hypothesized that YF2359 might have an unusual mutation.
Table 1.
Carotenoid contents (𝜇g·g-1 fresh weight) of the ripe fruit of M82, YF2359, and E6203 quantified by HPLC analysis
Linkage Analysis of the Yellow Fruit in YF2359
To identify the linkage between the yellow fruit and genes in YF2359, we constructed a linkage map for 235 F2 individuals (YF2359 × LA1589). To construct the linkage map of the yellow fruit, 30 InDel and dCAPS markers that spanned the tomato genome were developed between S. lycopersicum and S. pimpinellifolium. Among them, two InDel markers (CH3-7 and CH3-8) have been described (Liu et al., 2017) (Suppl. Table 1s.). The yellow fruit of YF2359 was found to be closely linked with the CH3-8 marker on chromosome 3. However, the CH3-8 marker was linked with PSY1 (Fig. 1B), so we developed a gene-specific dCAPS marker (CH3-6 marker) in PSY1. We constructed the additional linkage map using six markers, including the CH3-6 marker on chromosome 3. The yellow fruit was mapped to a 28.1-cM region and cosegregated with PSY1 (Fig. 1B). Using the CH3-6 marker, we identified 69 and 110 homozygous and heterozygous red-fruited progeny, respectively, and 56 homozygous yellow-fruited progeny corresponding to the expected ratio of 1:2:1 (Fig. 1C and Table 2). In total, 179 and 56 progenies had red and ripe yellow fruit in the F2 population, respectively. The segregation ratio of fruit color phenotypes in the F2 population did not deviate from the expected 3:1 (red:yellow) ratio (Table 2). Thus, inconsistent with our initial hypothesis, the yellow fruit of YF2359 was cosegregated with PSY1, so we decided to analyze the PSY1 sequence in YF2359 comprehensively.
Table 2.
Segregation of the PSY1 genotype and ripe fruit color in 235 F2 individuals derived from a cross between YF2359 (Solanum lycopersicum) and LA1589 (Solanum pimpinellifolium)
| Expected ratio | Observed frequency | χ2-test | p-value | |
| Phenotype | 3:1 | 179 (red):56 (yellow) | 0.17nsz | 0.9178 |
| Genotype | 1:2:1 | 69 (-/-):110 (-/r):56 (r/r) | 2.40ns | 0.3018 |
Analysis of the PSY1 Transcripts in YF2359
We sequenced the 3ʹ-UTR of PSY1 cDNAs from YF2359 fruit to assess whether they had a sequence variation. Two different PSY1 transcripts were detected in the fruit of YF2359. One transcript was identical to the wild-type PSY1 transcript in M82 and consisted of a 545-bp 5ʹ-UTR, a 1239-bp coding region, and a 742-bp 3ʹ-UTR. The other transcript was a fusion-type consisting of a 545-bp 5ʹ-UTR, a 1299-bp coding region, and a 514-bp 3ʹ-UTR (Fig. 2B). This transcript was produced by the fusion of exons from two different DNA strands, namely, the PSY1 strand and the antisense strand of CoA ligase. The fusion transcript had a portion of the sixth exon (76 bp) and deletion of the entire 3ʹ-UTR (742 bp), with a new 645-bp segment fused at the deletion site of the sixth exon (Fig. 2B). The new 645-bp segment was predicted to be produced from the antisense strand of CoA ligase and was complementary to the 5ʹ-UTR (445 bp), first exon (177 bp), and second exon (23 bp) of CoA ligase (Fig. 2A). An additional five nucleotides (TATGC) were also inserted at the deletion site. However, their origin is unclear because of the lack of a corresponding sequence in the complementary strand (Fig. 2B). The coding region of the wild-type and fusion-type encoded 412 and 432 amino acids, respectively. We observed that 25 amino acids in the fusion-type were substituted by 45 amino acids at the C terminus. Two conserved lysine residues in the trans-isoprenyl diphosphate (trans-IPP-HH) domain of PSY were substituted by asparagine and methionine in the fusion-type (Fig. 3A). Given that these two lysine residues are phylogenetically conserved (Fig. 3B), they are presumably essential for the function of PSY1, so the fusion mutation probably nullifies PSY1 activity.
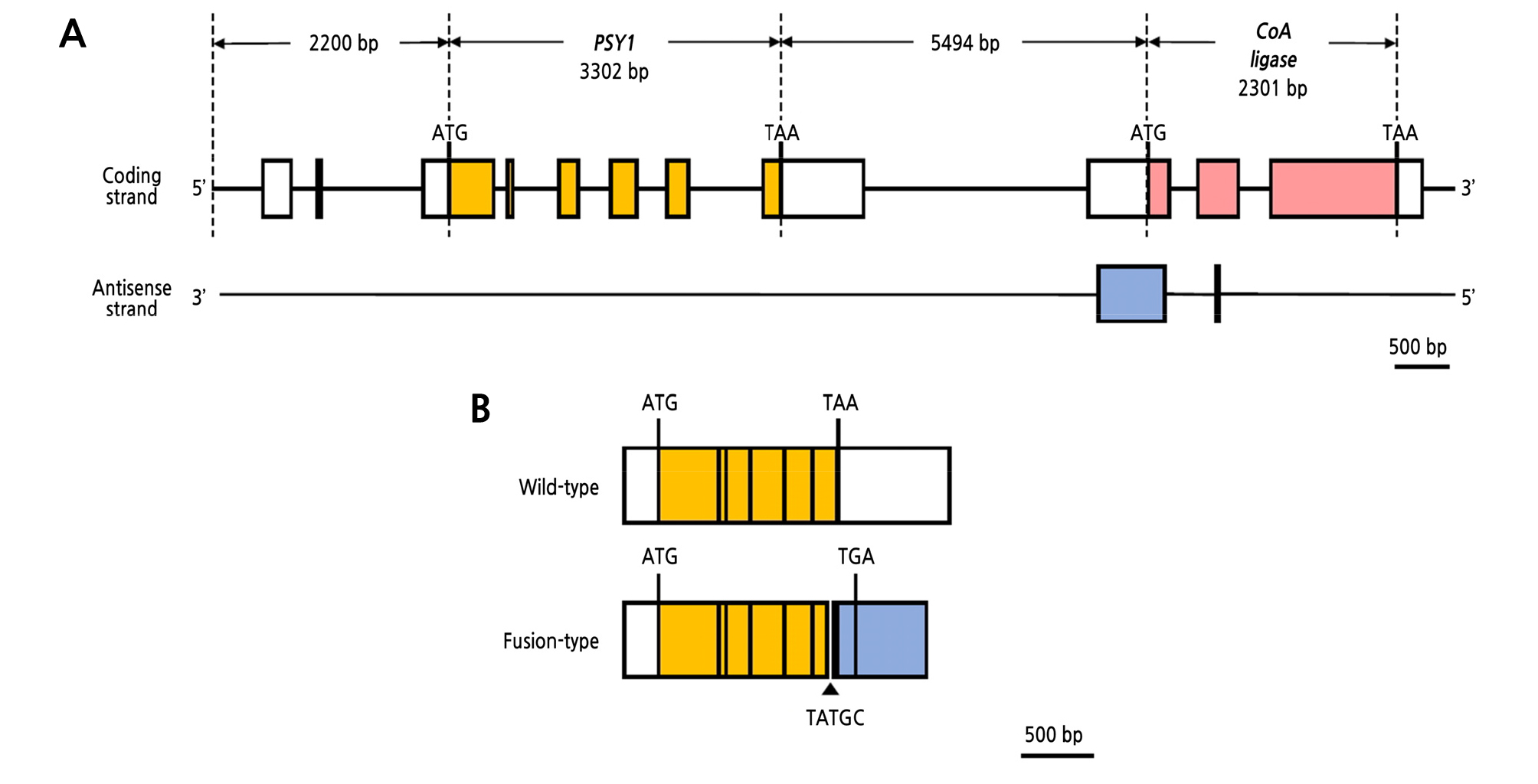
Fig. 2.
Schematic representation of the genomic structure of PSY1 in YF2359. (A) Schematic representation of the genomic structure of tomato PSY1 and CoA ligase in chromosome 3. Exons are shown by orange, pink, and blue boxes, and introns are represented by solid lines. White boxes represent the 5ʹ-UTR and 3ʹ-UTR. PSY1 consisted of 3302 nucleotides, including six exons and five introns. CoA ligase was composed of 2301 nucleotides, with three exons and two introns. The antisense nucleotides of CoA ligase were complementary to 445 bp of 5ʹ-UTR, 177 bp of the first exon, and 23 bp of the second exon of CoA ligase. (B) Schematic representation of the full-length cDNA structure of the two PSY1 transcripts in YF2359. The wild-type consisted of a 545-bp 5ʹ-UTR, a 1239-bp coding region, and a 770-bp 3ʹ-UTR. The fusion-type was composed of a 545-bp 5ʹ-UTR, a 1299-bp coding region, and a 514-bp 3ʹ-UTR. In the fusion-type, a portion of the sixth exon (76 bp) and the entire 3ʹ-UTR (742 bp) were deleted, and 645 nucleotides from the antisense strand in CoA ligase were fused at the deletion site with an additional five nucleotides (TATGC). The stop codon was altered from TAA to TGA in the fusion-type.
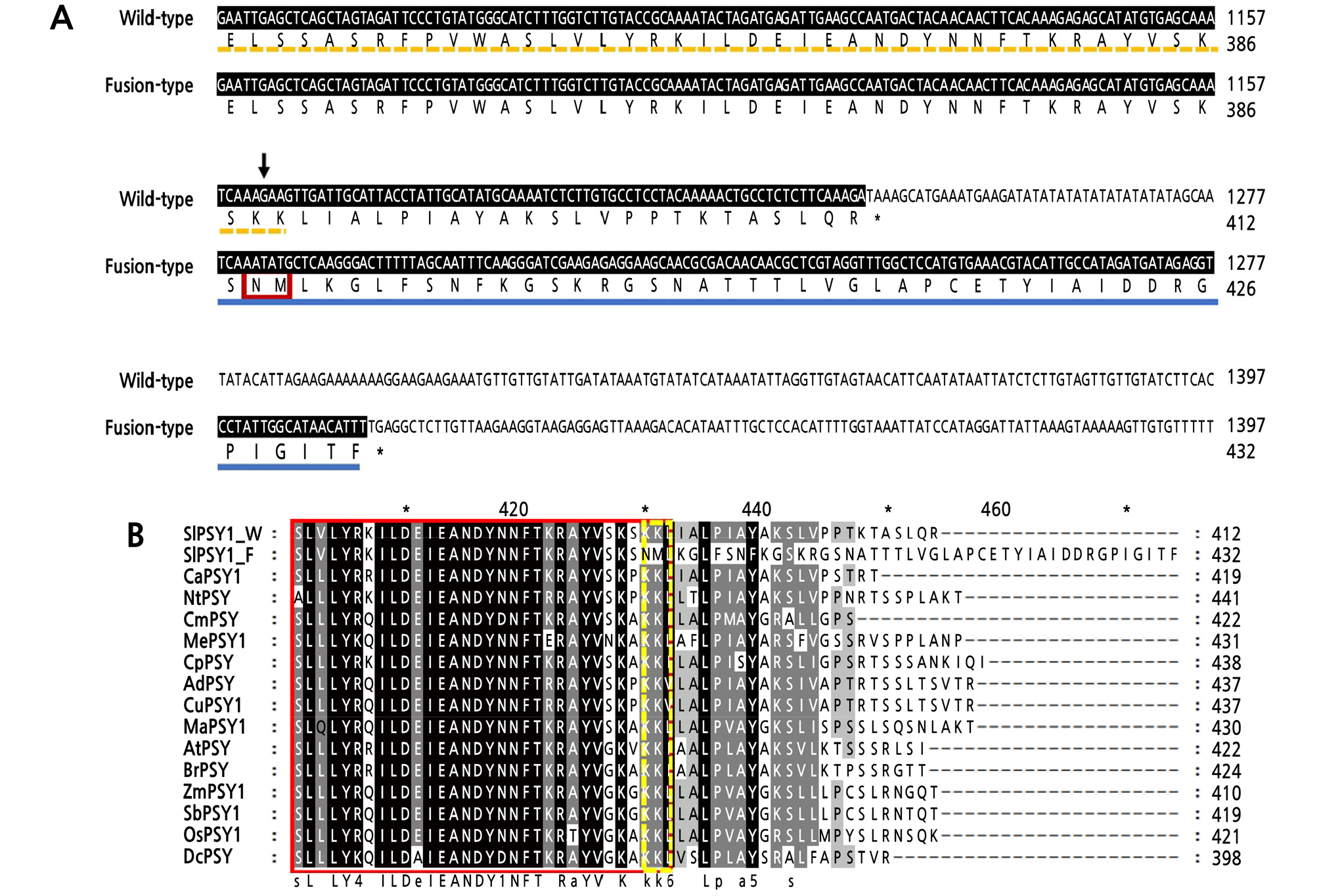
Fig. 3.
Comparison of cDNA and protein sequences between the two PSY1 transcripts. (A) Comparison of the 3ʹ end of the cDNAs and altered amino acid sequences between the two transcripts of PSY1, namely, wild-type and fusion-type, in YF2359. cDNA sequences were different from the arrow point. Different amino acids are underlined in blue in the fusion-type. The stop codons are shown in red font. The trans-IPP-HH domain is underlined by a yellow dotted line in the wild-type, and the substitution of amino acids in the trans-IPP-HH domain is boxed in red in the fusion-type. (B) Multiple sequence alignment of the PSY1 protein in various species. Protein sequences were aligned using ClustalX 2.1. The red box indicates the part of the trans-isoprenyl diphosphate synthase (trans-IPP-HH) domain. The yellow dashed line box indicates the different sequences between fusion-type and various species in the trans-IPP-HH domain. SlPSY1, both wild-type (W) and fusion-type (F), was sequenced in this study. The PSY1 sequences are as follows: CaPSY1 (ACE78189.1) from Capsicum annuum; NtPSY (ADK25054.1) from Nicotiana tabacum; CmPSY (AEH03200.1) from Cucumis melo; MePSY1 (ACY42664.1) from Manihot esculenta; CpPSY (ABG72805.1) from Carica papaya; AdPSY (ACO53104.1) from Actinidia deliciosa; CuPSY1 (AF220218) from Citrus unshiu; MaPSY1 (JX195664) from Musa acuminata; AtPSY (NP_001031895) from Arabidopsis thaliana; ZmPSY1 (AAR08445) from Zea mays; SbPSY1 (AY705389) from Sorghum bicolor; OsPSY1 (AAS18307) from Oryza sativa; DcPSY (DQ192186) from Daucus carota.
Expression Analysis of the Carotenoid Biosynthesis Genes in YF2359
The expression level of the two PSY1 transcripts, that is, the wild-type and fusion-type, was analyzed by qRT-PCR in red (M82 and E6203) and yellow fruit (LA4044 and YF2359), respectively. The expression level of the wild-type transcript in YF2359 was significantly lower (1000-fold) than that in M82. The level of the fusion-type transcript in YF2359 was comparable to that in wild-type M82, which did not express a detectable level of the fusion-type. In addition, the expression of the fusion-type was higher than the wild-type in YF2359 (Fig. 4A). These results indicate that the fusion mutation renders the wild-type PSY1 expression in YF2359 much lower than that in M82. Furthermore, the fusion-type was not detected in E6203 and LA4044 (Fig. 4A) even though the LA4044 has been known as yellow flesh (r) with PSY1 mutation (Tomato Genetic Resource Center, USA). These findings suggest that the fusion-type was a PSY1 transcript detectable only in YF2359.
We also evaluated the expression level of the genes involved in the carotenoid biosynthesis pathway in M82 and YF2359. Except for ß-carotene hydroxylase 2 (CrtRb2), the expression level of the carotenoid biosynthesis genes was significantly lower in YF2359 than in M82 (Fig. 4B). Therefore, the nonfunctional fusion mutant presumably renders a lower expression of wild-type PSY1, in turn,perturbing the carotenoid biosynthetic flux, resulting in ripe yellow fruit with low carotenoid contents in YF2359 (Fig. 1A and Table 1).
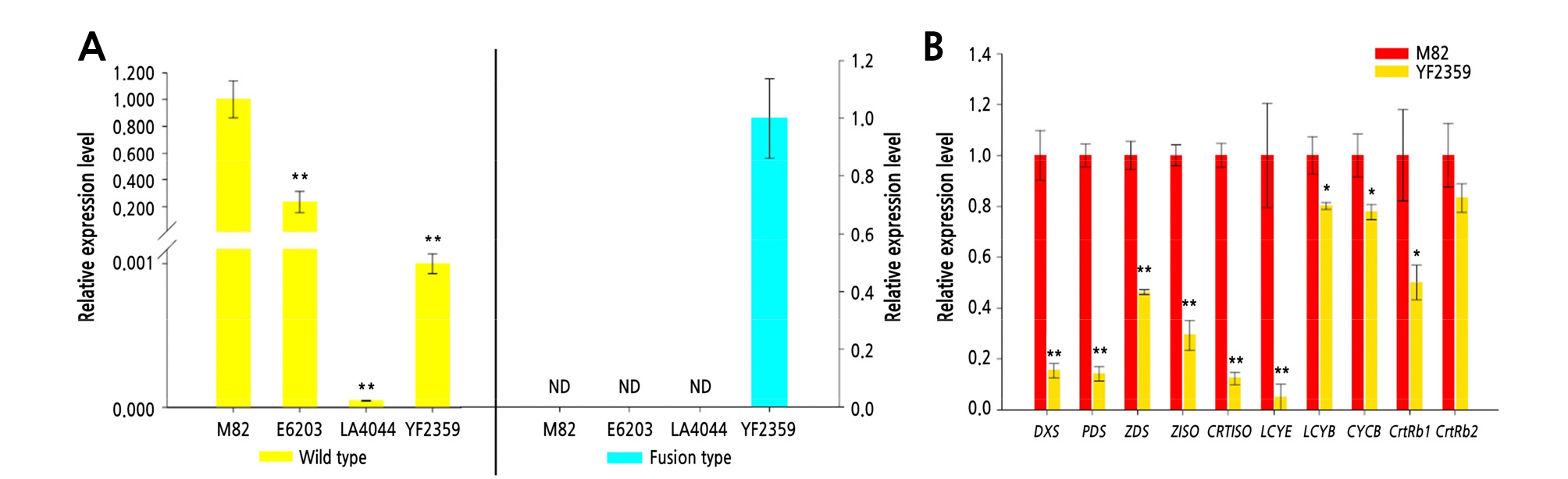
Fig. 4.
Expression analysis of the carotenoid biosynthetic genes by qRT-PCR. Expression level of carotenoid biosynthetic genes was analyzed in ripe fruit (10 days after the breaker stage). (A) The relative expression levels of the two transcripts of PSY1 in M82, E6203, LA4044, and YF2359. There was no detectable fusion transcript in M82, E6203, and LA4044. The expression level of wild-type PSY1 transcript in YF2359 was significantly lower than that in M82, whereas the fusion-type expression in YF2359 exhibited a similar level as the wild-type expression level in M82. * and ** indicate significant difference at p < 0.05 and p < 0.01 by Dunnett’s test (n ≥ 3). (B) The relative expression levels of carotenoid biosynthetic genes in M82 and YF2359. DXS, 1-deoxy-D-xylulose 5-phosphate synthase; CYCB, chromoplast-specific lycopene β-cyclase; CRTISO, carotenoid isomerase; CrtRb1, chloroplast-specific β-ring hydroxylase; CrtRb2, chromoplast- specific β-ring hydroxylase; LCY-B, chloroplast-specific lycopene β-cyclase; LCY-E, lycopene ε-cyclase; PDS, phytoene desaturase; PSY1, phytoene synthase; ZDS, ζ-carotene desaturase; ZISO, ζ-carotene isomerase. ACTIN was used for normalizing the expression level. * and ** indicate significant difference at p < 0.05 and p < 0.01 by t -test (n ≥ 3).
Discussion
To determine the genetic factor underlying the yellow fruit of YF2359, we conducted a linkage analysis of this trait. The recessive trait was mapped to a 28.1-cM region covering PSY1 on chromosome 3 (Fig. 1B and Table 2) and the yellow fruit trait cosegregated with PSY1.
The fruit color of tomato is associated with accumulation of carotenoids encoded by the PSY1 gene during ripening (Lado et al., 2016). PSY1 is a crucial enzyme involved in the first committed step of the carotenoid biosynthetic flux to produce phytoene for synthesizing carotenoids in tomato (Liu et al., 2015; Sun et al., 2018) Overexpression of PSY1 gene enhanced carotenoid content in fruit, whereas there is no carotenoid accumulation in the ripe fruitwith knock-out of the PSY1 gene in tomato (Gady et al., 2012). In YF2359, which has the yellow flesh phenotype, carotenoid accumulation was much less than in the red-fruited tomato M82 (Table 1). In addition, the carotenoid profile of YF2359 was similar to that of the yellow flesh (r) mutant (Shin et al., 2019). This result implies that YF2359 may have a defect in carotenoid accumulation, resulting in the yellow fruit.
The fruit-specific expression of PSY1 was substantially increased during fruit ripening (Cao et al., 2019). A previous study also reported a concomitant increased transcriptional level of PSY1 and carotenoid contents during fruit ripening (Pandurangaiah et al., 2016). Here, we identified that there were two PSY1 transcripts, namely, wild-type and fusion-type, in YF2359 (Fig. 2B). The expression level of the fusion-type was higher than that of the wild-type in the ripe fruit of YF2359. In addition, although the expression of the fusion-type in YF2359 showed a similar level as the wild-type PSY1 in M82, the wild-type PSY1 level was much lower in YF2359 than in M82 (Fig. 4A). This result suggests that the fusion-type impaired the accumulation of the wild-type transcript because of a shared region of PSY1 between the two transcripts (Fig. 2B). Therefore, the reduced wild-type transcript level may result in deficient production of phytoene synthase required for the normal level of carotenoid biosynthesis, leading to the ripe yellow fruit of YF2359 (Fig. 1A and Table 1).
Several mechanisms regulate the carotenoid biosynthesis pathway, such as the control of gene expression at the transcriptional level, post-transcriptional regulation, the substrate specificity of enzymes, and feedback regulation. In the feedback regulation, a lack of β-carotene causes increased activity of the up-stream enzymes in the carotenoid biosynthesis pathway (Diretto et al., 2020). Accordingly, a high level of lycopene has been detected in the old-gold crimson tomato mutant, which is deficient in β-carotene because of a mutation in LCYB (Bramley, 2002). Prolycopene and neurosporene produced by ζ-carotene desaturase are predicted to affect PSY1 transcription by feedback regulation. In t3002/r2997 double mutant tomato, PSY1 transcription is partially restored by prolycopene, so sufficient amounts of phytoene and down-stream carotenoids are produced (Kachanovsky et al., 2012). We predicted that down-stream carotenoids of the carotenoid biosynthetic flux, such as β-carotene, may affect the PSY1 transcription in YF2359 by negative feedback regulation. However, the underlying molecular mechanism is not well established and requires further research.
PSY is the major rate-limiting factor responsible for the pool size of carotenoids in plant (Sun et al., 2018). Highly conserved residues are important for PSY activity. For example, mutations of the key residues in PSY structures significantly decreased PSY activity (Cao et al., 2019). PSY1 contains a trans-IPP-HH synthase domain between the Asp122 and Lys389 residues and is predicted to affect catalytic activity and substrate recognition (Supathaweewat and Klanrit, 2013). We identified that the PSY1 fusion mutant had amino acid substitutions in the trans-IPP-HH domain (Fig. 3A). Two conserved lysine residues were substituted for asparagine and methionine at the C-terminal end of the fusion-type transcript (Fig. 3B). Given their phylogenetic conservation, these two amino acids may be crucial for functional PSY1, which could explain the fusion mutation that may nullify the PSY1 function in YF2359. These data indicate that in addition to the PSY1 catalytic activity, the fusion-type may not recognize GGPP as the substrate required for the production of phytoene. Therefore, the reduced phytoene synthase level hinders the carotenoid biosynthesis and results in yellow fruit.
Through sequencing analysis, the wild-type was determined to be identical to the PSY1 transcript of M82, whereas the fusion-type consisted of PSY1 and the antisense strand of CoA ligase, suggesting the post-transcriptional regulation of PSY1 in YF2359 (Fig. 2).The sequence difference between the two transcripts was expected due to trans-splicing in YF2359. Genic trans-splicing is a type of trans-splicing that results from the combination of parts of two different mRNA transcripts joined at the splice site (Lasda and Blumenthal, 2011). This type of trans-splicing involves exons produced from different pre-mRNAs of the same gene or transcripts of different genes or intergenic regions. Such trans-splicing events have been found in the modifier of mdg4 of Drosophila (Labrador et al., 2001). However, trans-splicing in plants has not been well studied. Here, we identified that the fusion-type transcript was generated by the joining of exons from two different strands,PSY1 and the antisense strand of CoA ligase (Fig. 2). Therefore, the fusion-type transcript in YF2359 appears to be produced by genic trans-splicing. Furthermore, the trans-splicing in PSY1 led to reduced expression of wild-type PSY1, implicating perturbation of the metabolic flux in the carotenoid pathway and resulted in the yellow flesh phenotype of YF2359. The results of studying this unusual mutation in PSY1 by trans-splicing that alters the fruit color of tomato will be helpful to understand other genetic variations in plants.
Supplementary Material
Supplementary materials are available at Horticultural Science and Technology website (https://www.hst-j.org).
- HORT_20200064_Table_1s.pdf
Primers used in this study
- HORT_20200064_Fig_1s.pdf
Fruit firmness of M82 and YF2359 in ripe fruits. The firmness was measured using a SUN RHEO METER COMPAC-100Ⅱ (Sun Scientific Co., Ltd.) fitted with a 3 mm plunger. Each fruit with eliminated peel was compressed to 10 mm on the equatorial zone. The data present the mean with standard error (n = 14).
- HORT_20200064_Fig_2s.pdf
Alignment for promoter and coding regions of PSY1 between M82 and YF2359. (A) Comparison of the promoter region sequences between the Heinz1706 and YF2359. (B) Comparison of the coding region sequences between the M82 and YF2359.




