Introduction
Materials and Methods
Experimental Site and Soil Sampling
Planting Kimchi Cabbage and Use of Plastic Mulch
Nematode Extraction and Counting
Temperature and Moisture Content
Statistical Analysis
Results
Nematode Population from Autumn 2015 and Spring 2016
Temperature and Moisture Content from Autumn 2015 and Spring 2016
Discussions
Introduction
Heterodera trifolii, more commonly known as clover cyst nematode, is currently a serious problem for Kimchi cabbage growers of the highland area in Korea (Mwamula et al., 2018). The nematode is widely distributed throughout the world with a wide host range that includes white and red clover, spinach, strawberry, cabbage, and radish plants (Evans et al., 2003). Due to limited information about the nematode on Kimchi cabbage in Korea, the pest steadily spread within the highland areas and has become a serious setback.
As vegetables or processed food, Kimchi cabbage [Brassica rapa pekinensis (Lour.) Rupr.] has a high market value and is ranked as one of the most produced vegetable crops in Korea (Chang et al., 2008; Kim et al., 2014). It is widely cultivated in the highland area of Korea and the main production constraints include: insect pests, diseases caused by various pathogenic microbes, and the recently identified H. trifolii (Kim et al., 2014; Kabir et al., 2018; Mwamula et al., 2018). Currently, crop losses caused by H. trifolii have only been quantified for clover, beets, and other leguminous crops (Perry and Gaur, 1996). In Korea, infestations of Kimchi cabbage fields by the nematode can cause up to 50% yield losses (Kabir et al., 2018) and have other effects on crop production (Kwon et al., 2016). Thus, the economic damage caused by H. trifolii is a major production setback and demands the development and implementation of reliable control and management strategies.
Since the nematode has been recently recognized as a new pest of Kimchi cabbage in Korea, effective management control strategies have yet to be established. Current management of H. trifolii involves an integrative approach of different strategies, which include: prevention, cultural practices, resistant cultivars, trap crops, physical methods and chemical control (Turner and Rowe, 2006). Chemical control is considered a more potent control method depending on soil properties but control of endoparasitic nematodes with chemicals is more difficult as nematicidal compounds have to be non-phytotoxic and preferably systemic (Gowen,1997).
The use of well-known, effective fumigants like 1, 3-dichloropropene is currently under restriction due to their side effects on non-target organisms and groundwater contamination (Urwin et al., 2000). Thus, an integrative approach is a more- favored alternative, and in planning for an integrative nematode management strategy, the use of physical methods has been documented to be feasible. Physical nematode control strategies include the use of heat, solarization in the form of polyethylene or plastic film mulching, and flooding to kill or limit nematode reproduction and development.
As an alternative to using nematicides or pesticides, plastic mulch is commonly used for growing vegetables (e.g., cucumber, cabbage, radish, and oriental melon). Plastic mulch can alter soil temperature and extend the cropping season into cooler or warmer seasons for example; black plastic mulch is often used in the spring to warm root zones temperatures (Cookey et al., 2016). Plastic mulches are used in many horticultural crops to raise soil temperature, suppress weeds, and conserve soil water (Brault et al., 2002). Mulching can effectively control weeds and enhance water and fertilizer management to ensure good harvest (Fortnum et al., 2000). Temperature has also similar importance in determining whether a given nematode species has the potential to establish in a new environment (Chakraborty et al., 2000). Various studies have shown that nematode population reductions can be achieved for species belonging to Meloidogyne spp., Heterodera spp., Pratylenchus spp., Globodera spp., Paratrichodorus spp., and others using soil solarization techniques (Stapleton and DeVay, 1986; Chellemi et al., 1997). However, the technique is expensive and not popular in Korean agriculture.
Currently, management practices of the pest on Kimchi cabbage in the highland (Jungsun, Ganwon-do province) areas are limited to prevention of spread and multiplication through proper disposal of infested waste soil and sanitation to avoid transfer of cysts to non-infested fields through farm machinery (Kwon et al., 2016). Thus, there is no readily available reliable management strategy to deal with infested fields. There is therefore a need to select and test more feasible management strategies, which are also environmentally friendly, for use in the control of the nematode. Thus, this study aimed at investigating the effect of plastic mulch on H. trifolii reproduction and multiplication on Kimchi cabbage in field conditions.
Materials and Methods
Experimental Site and Soil Sampling
Experiments were conducted in two different planting seasons (autumn 2015 and spring 2016) in a highly nematode infested Kimchi cabbage field at Jungsun of Gungwondo province, Korea. The geographical location of the site is 37 ° 26' 23.20"N and 128° 51' 23.80"E. The average altitude of the area is 536 m above sea level. The site was originally a cultivated land, traditionally tilled for planting Kimchi cabbage, pH - 7.19, Soil electrical conductivity EC - 0.35 (dS·m-1), organic matter - 22.2 (g·kg-1), Cations and cation exchange capacity CEC - 9.76 (cmol·kg-1), soil texture - sandy loam (sand 62.3%, silt 19.2%, clay 18.5%), and noted to be infested with clover cyst nematode. For both seasons, soil samples were obtained before planting and 60 days after planting. For the autumn season, Kimchi cabbage was planted on 14 August 2015. For the following spring season, cabbage was planted on 4 May 2016. Samples were collected on 13 October 2015 (autumn) and 2 July 2016 (spring), respectively. Each sample was taken from a single plant root surface, approximately 5 cm in diameter and 15 cm in depth (standard U.S. Department of Agriculture nematode sampling method) using a transplanting trowel. All collected samples were taken to the laboratory for further analysis.
Planting Kimchi Cabbage and Use of Plastic Mulch
After land preparation, the field (20 × 30 m) was divided into three different rows. Two rows were covered with either black or transparent mulches (plastic mulches used for this experiment were manufactured by Sewon; film was made of polyethylene; black- thickness: 0.012 mm, width: 90 mm; transparent- thickness: 0.015 mm, width: 90 mm) while the third row was left bare (as a control). Consequently, three covers (i.e., black mulch, transparent mulch, or bare soil were prepared at a length of 10 m and a width of 1.5 m, with three replicates of each cover. 30-day-old Kimchi cabbage plants (‘CR-norang’ as the autumn cultivar and ‘Chungwang’ as the spring cultivar) were purchased from a local market and planted in the experimental plot (12 plants per row). All plants were subjected to watering (40 L per row during planting and when required thereafter) and application of starter fertilizer (Deogichan M compound fertilizer NPK, 200 g per row).
Nematode Extraction and Counting
Initially, plant shoots (regarded as the portion of the plants above the ground level) and soil with roots (500 - 800 g) were collected as samples and taken to the laboratory for further analysis. H. trifolii cysts from the collected soil samples were isolated by sieving 300 g of soil. Soil with roots was washed carefully using tap water and the resultant water was passed through (20 -, 60 -, and 400 - mesh sieves). Cysts were collected on a 60-mesh sieve and were filtered with Whatman no. 100 filter paper to remove the water content. Cysts on the filter paper were then observed under a stereomicroscope (SM2 1000, Nikon, Japan) to count total cysts and cysts with eggs. Five cysts (undamaged cysts with eggs) were transferred into a small vial in 5 mL water. The cysts were sonicated at 800 rpm using a Polytron PT 1300D sonicator (Kinematica AG, Switzerland) and counted under a stereomicroscope to obtain the number of eggs/cyst and the total number of eggs.
Temperature and Moisture Content
Soil, air temperature, and soil moisture content of the two mulches and the bare soil were automatically recorded using data loggers (WatchDog 1450 data logger, Spectrum Technologies, Inc., USA) in 24-hour intervals until harvesting. WatchDog data loggers were placed in the experimental plot on the mulch/soil covers and data were collected while sampling. The mean temperature for seven days was calculated based on these data. The temperature frequency were expressed as the total number of hours with temperature values that occurred within specific range (from 0°C to 37°C, in steps of 1°C).
Statistical Analysis
The data were analyzed by an analysis of variance using SAS 9.4 (SAS Institute Inc., NC, USA). Initial and final numbers of total cysts, cysts with eggs, eggs/cyst, and total eggs were compared between the different mulch/soil covers. Fisher’s least significant difference test was conducted with significance set at p ≤ 0.05.
Results
Nematode Population from Autumn 2015 and Spring 2016
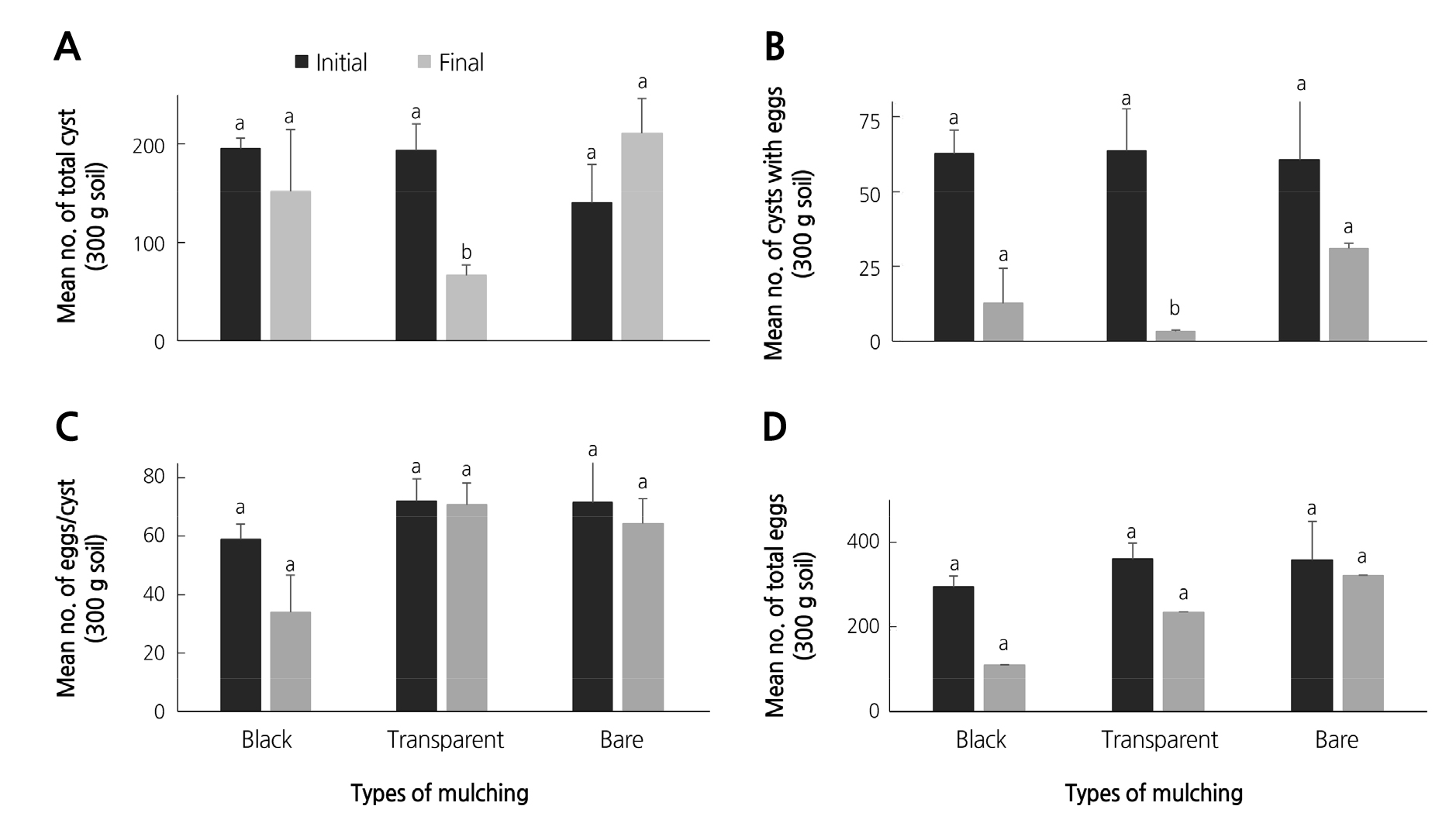
All final nematode populations (total cysts, cysts with eggs, females, eggs/cyst, and total eggs) obtained from the different mulches resulted in varying numbers. Noteworthy, both black and transparent mulches resulted in decreased final populations of H. trifolii compared to bare soil (Fig. 1). The total cysts (Fig. 1A), cysts with eggs (Fig. 1B), and total number of eggs from the transparent mulch were significantly lower compared to the black and bare soil populations (p values were 0.012, 0.012, and 0.05, respectively). However, the final population of eggs/cyst from the transparent mulch were not significantly lower than the initial population (Fig. 1C). Surprisingly, no females were recorded from the nematode populations.
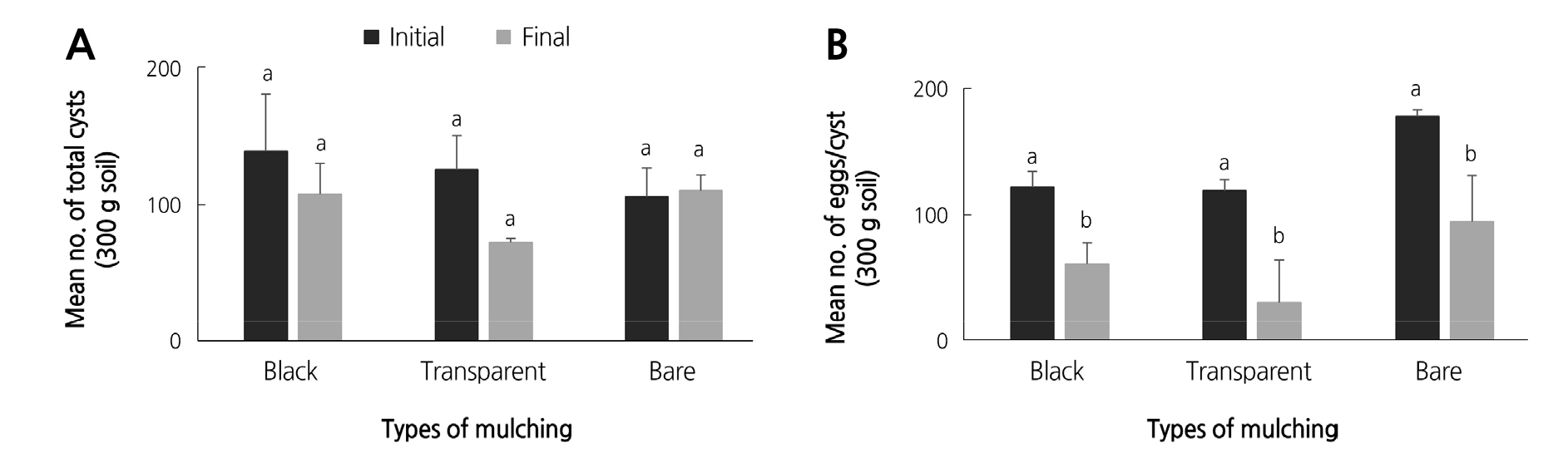
Although all final nematode populations (total cysts, cysts with eggs, and eggs/cyst) obtained in spring 2016 from the different mulches decreased compared to the initial populations, the nematode population differences between the different mulches were not statistically significant. Total cysts from the transparent mulch showed only slightly non-significant differences (p = 0.07). The p value of total cysts from the black and bare soil was 0.53 and 0.85 respectively (Fig. 2A-B).
Noteworthy, cysts with eggs (Fig. 2B) from the two mulches and the bare soil showed highly significant differences between them (p values were 0.005, 0.007, and 0.005, respectively). The mean number of eggs/cysts also decreased by half relative to populations from the previous season (Fig. 1C).
Temperature and Moisture Content from Autumn 2015 and Spring 2016
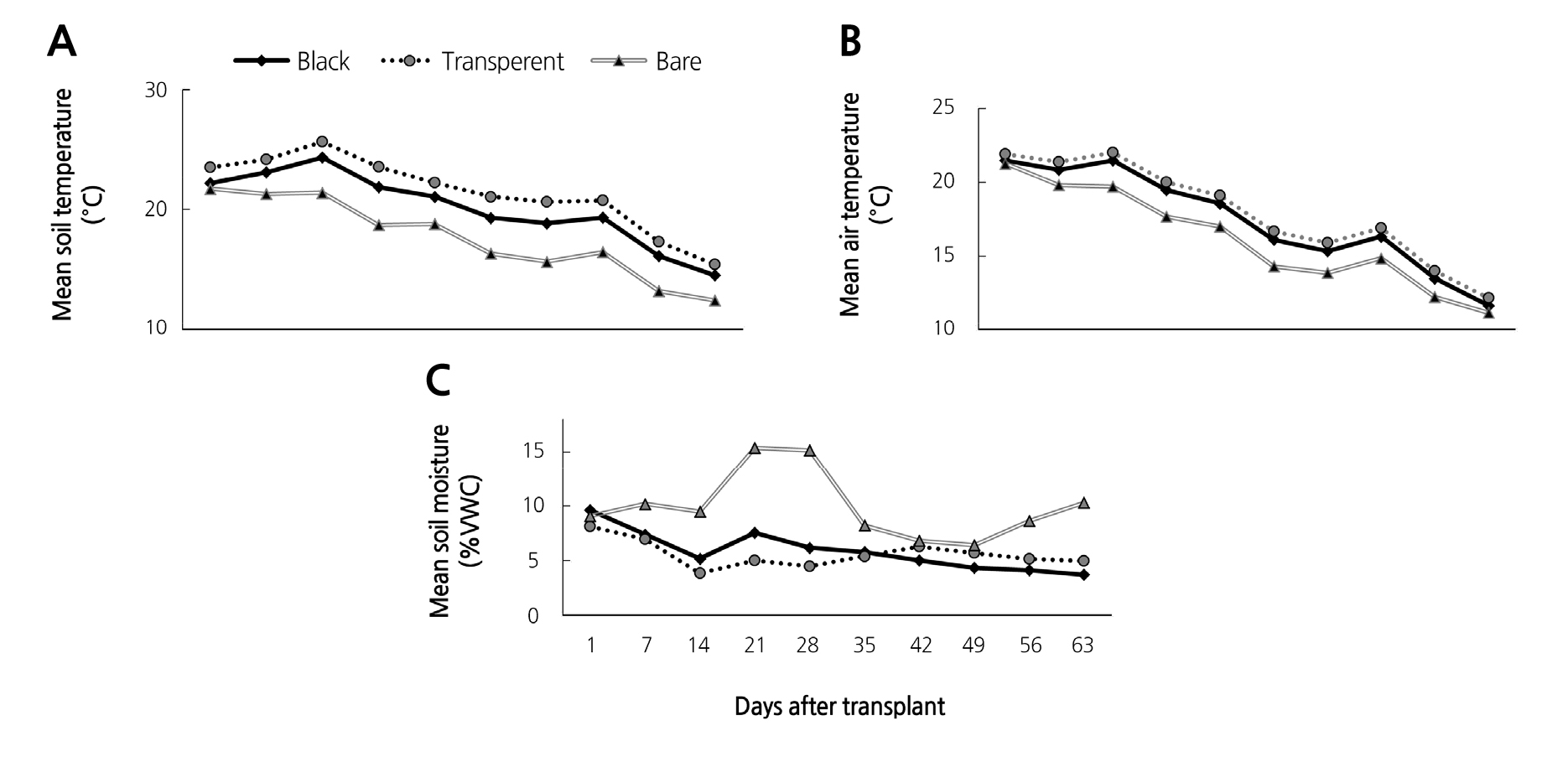
Mean soil and air temperature showed a consistent pattern of relationships: the transparent mulch resulted in the highest soil temperature in comparison with the black mulch and bare soil (Fig. 3A). However, no apparent differences were noted for air temperature between the black and transparent mulches (Fig. 3B).
Soil moisture clearly depicted a different pattern than that of soil and air temperature (Fig. 3C). Initially, after the first two weeks, mean moisture content from the black and transparent mulches was below 6%, whereas moisture content from the bare soil was above 15% between the third and fourth weeks after planting. After five weeks, soil moisture from the two mulches and bare soil appeared to give similar values, but bare soil increased again until the end of the experiment. Maximum soil moisture content was recorded from the bare soil; however, these differences were not statistically different.
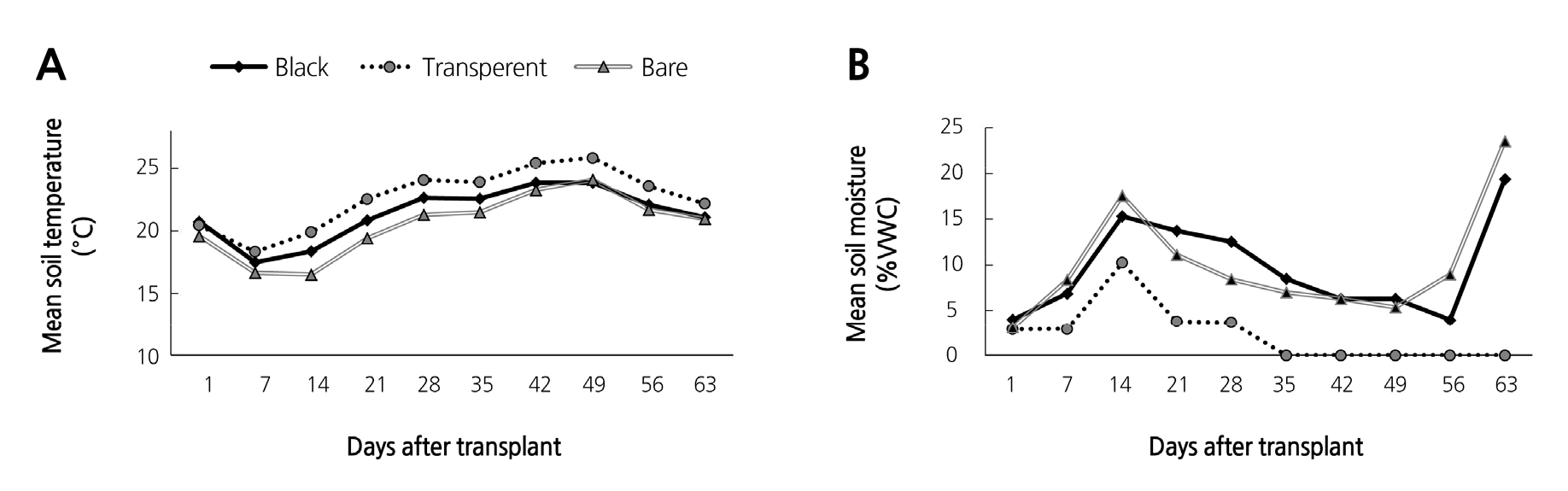
In spring 2016, soil and air temperature did not show any significant differences between mulches, however, soil temperature from the transparent mulch was slightly higher than the black mulch (Fig. 4A). In contrast, air temperature from black and transparent mulches was no different than that of the bare soil.
Soil moisture content (Fig. 4B) showed a similar pattern to the autumn 2015 moisture data. Transparent mulch showed very low moisture content in comparison with the black mulch and bare soil. Initially, the moisture content from the bare soil was 17.57% on the 14th day. Afterward, the black mulch displayed the highest moisture content up to the 50th day. Again, the moisture content from the bare soil was higher than that of the black mulch. Additionally, the transparent mulch moisture content was significantly lower than that of the other two (black mulch and bare soil). During the last week, soil moisture from the bare soil and black mulch reached up to 23.57 and 19.37%, respectively.
Discussions
Apparent at both seasons, covering soil with either black or transparent mulch resulted in decreased final populations of H. trifolii compared to bare soil. It is generally known that soil solarization plays a vital role in nematode population reductions through a hydrothermal effect, which becomes more pronounced at higher temperatures (Viaene et al., 2006; Aminu-Taiwo et al., 2014). In cooler periods, the process is not effective (Mahrer et al., 1987). However, both the cabbage and the pathogen are highly sensitive to temperature (Kabir et al., 2018) and the rising soil temperatures may exert a selection pressure among beet cyst nematodes to adapt to changes in temperature (Kakaire et al., 2012). Studies on thermal deaths have shown that reductions in populations of different soil pathogens, including nematodes, can occur after 4 - 6 weeks at 35 - 50°C (Viaene et al., 2006). Use of fertilizers in nematode-infested fields may considerably decrease nematode populations (Zhang et al., 2009). Transplanting the plant seedlings was found as an effective screening method to suppress the root knot nematode (Meloidogyne incognita) in tomato and chili in Korea (Hwang et al., 2013, Hwang et al., 2016). Likewise, there is substantial evidence that the addition of organic matter in the form of compost or manure can decrease significant amount of nematode pest populations (Kodira et al., 1995). In addition, plastic mulches are used in the growing of many horticultural crops to raise soil temperature, suppress weeds, and conserve soil water (Brault et al., 2002).
Surprisingly, the final number of cysts with eggs from the bare soil (autumn 2015) also decreased, similar to what was observed for black and transparent mulch (Fig. 1B). This can probably be explained by the lower temperature condition followed by a relatively high temperature. The meteorological data of Jungsun during autumn 2015, showed decreasing temperature condition starting from the month of September (KMA, 2011). Our temperature data also showed a similar pattern (Fig. 3A-B). Additionally, the final number of eggs/cyst from the transparent mulches was much lower compared to black mulch and bare soil (Fig. 1D). Past studies revealed that, low temperature inhibits egg hatching and the emergence of second stage juveniles in H. schachtii and hatching may be equally inhibited by a period of warmth following low temperatures (Shepherd and Clarke, 1971), a condition known as delayed dormancy (Zheng and Ferris, 1991). This delayed dormancy could make the cyst viable until the next favorable temperature condition and may explain why the eggs/cyst from the final population showed an increase over the initial population in autumn 2015. We assume that under field conditions, egg maturation of this nematode is favored by autumn temperature. However, the decreasing temperatures towards winter could initiate a state of dormancy. In connection, transparent mulching appeared to result in maximum soil and air temperature (Fig. 3A, B), whereas bare soil showed the lowest temperature. However, transparent mulching resulted in lower soil moisture content in comparison with the black mulch and bare soil (Fig. 3C). Using soil solarization technique, a varied number of studies have shown population reductions of various nematode species belonging to different genera such as Meloidogyne spp., Heterodera spp., Pratylenchus spp., Globodera spp., and Paratrichodorus spp. (Stapleton and DeVay, 1986; Chellemi et al., 1997; Fortnum et al., 2000). As the transparent mulch resulted in higher temperature and lower moisture content, the dry condition of the soil might trigger cysts to enter the obligate diapause stage, as stated by Zheng and Ferris, (1991). This might be the reason why the final population of eggs/cyst showed was similar to the initial.
Different stages of H. trifolii populations in different mulches also showed lower final populations in spring 2016. Fig. 2A and 2B showed that the final number of total cysts and eggs/cyst from transparent mulch were reduced significantly relative to the other mulches. In comparison to the autumn 2015 nematode population, the total cysts from spring 2016 decreased at least one-fold. Surprisingly, the mean number of eggs/cyst from all mulches in 2016 doubled in comparison to 2015. In field condition, the temperature rose up to 35°C (KMA, 2011) and is mainly known as a temperature increasing condition. However, in the Korean highlands, maximum emergence of second stage juveniles generally occurs in late May (data not published), when the average air temperature ranges from 14 to 20°C (KMA, 2011). Increasing temperature conditions in the spring 2016 under transparent mulch may have contributed to higher eggs/cyst in comparison with 2015. Increasing temperatures are thus likely to cause higher levels of nematode hatching, potentially resulting in higher infestation levels (Kabir et al., 2018). In addition, Kakaire et al. (2012) stated that rising soil temperatures might exert a selection pressure among beet cyst nematodes to adapt to changes in temperature. Plastic mulch can increase the soil temperature and soil moisture, which is often exploited to increase the root zone temperature. Like temperature and moisture, plastic mulch can also alter the microclimate, which can suppress weed and soil pathogens (Fortnum et al., 2000). Although, the increasing temperature condition could have a positive effect on the growth of Kimchi cabbage, the condition is also good for nematode hatching. Nevertheless, the successful hatching of most of the cyst nematodes seems to largely depend on the soil environment, as these organisms spend long periods in the soil (Koenning and Sipes, 1998).
In spring 2016, soil temperatures and soil moisture (Fig. 4A, B) showed maximum recorded levels throughout the experimental period under transparent mulch. However, there was no notable significant differences between the mulches and bare soil. Mahadeen (2014) showed that black plastic mulches raise soil temperatures more quickly than other types. In line with this, the transparent mulches resulted in lower moisture content than for black mulch and bare soil. As the transparent mulch contained the lowest soil moisture, this could have resulted in maximum soil temperature and reduced final nematode populations. In general, the experiment showed that transparent plastic mulch was more effective in reducing H. trifolii populations in the soil compared to black mulch and bare soil. It has been noted in various experiments that transparent mulches are more effective in raising soil temperature in comparison with opaque or black plastic mulches (Streck, et al., 1996). This is because transparent mulches confer a relatively large net radiation at the soil surface and increase soil heat influx more efficiently than colored mulches (Streck et al., 1996; Dufour et al., 2003).
Our study results suggested that transparent mulching significantly reduces the final population of H. trifolii; however, black mulch can also reduce nematode populations. Mulching with plastic films effectively increases nutrient, water, and agrochemical use efficiency and leads to overall increases in harvest (Fan et al., 2005; Ibarra-jiménez et al., 2011). The field conditions tested in the experiment indicated that initial solar radiation exposure of the field is limited and progresses with time. Given the fact that an increase in soil temperature is the primary effect of soil solarization (Dufour et al., 2003), it is likely that the temperature within the given period was not high enough to create an impact on field-tested H. trifolii populations. Based on the the final population from both seasons, the total number of cysts from both mulches were significantly lower compared to the control condition. This could be explained by the fact that the transition period from spring (time of cabbage planting) to summer (time of harvesting) is characterized by significant increases in soil temperatures through increased thermal heat exposure. Soil temperatures between 40 - 50°C have been reported in solarization processes under prolonged solar radiation exposure (Katan and DeVay, 1991). Heterodera spp. favorably reproduces at temperatures of 21 - 27°C and beyond 32.5°C, development is limited (Melton et al., 1986). Increased temperatures thus could translate to both direct thermal killing of H. trifolii and the effect of sub-lethal heating below the soil depth on the growth rates and reproduction of the nematode (Katan and DeVay, 1991). Similar findings were shown for Meloidogyne hapla, where populations were virtually eradicated at depths at which thermal deaths could hardly be expected. Sub-lethal heating could have caused inhibition of subsequent egg hatching (Stapleton and DeVay, 1986).
Thus, the limited number of eggs per cyst of H. trifolii in the soil covered by transparent mulch could be associated with effects of a larger net radiation at the soil surface and increased soil heat influx. Additionally, Heterodera avanae was completely controlled by solarization except at the edge of mulch using transparent polyethylene sheets (Grinstein et al., 1995). Surprisingly, the infective juveniles recovered from autumn 2015 and spring 2016 were very negligible (data not shown). The reason for the lower number of viable cysts and infective juveniles could be explained by the fact that white mustard was planted as a trap crop in the fall as soon as the summer crop was harvested and the roots penetrated before the temperatures were low enough to inhibit nematode activity.
H. trifolii presents unique problems in management and the eggs inside their cysts may remain dormant for many years and may only hatch under favorable conditions (Riggs and Schuster, 1998). In conclusion, the effectiveness of solarization depends on available solar radiation during the treatment period and on soil thermal properties. Our study thus demonstrated that upon prolonged exposure of the soil to solar radiation, transparent mulching reduces nematode reproduction. The transparent mulch is therefore recommended as a potential non-chemical measure in controlling H. trifolii in the highland area of Korea.