Introduction
Materials and Methods
Plant Material and Experimental Design
Phenological Stages of Kumquat
Shoot Growth Pattern and Measurements
Flowering and Fruiting Measurements
Statistical Analysis
Results
Phenological Stages of Kumquat
Shoot Growth Pattern and Measurements
Flowering Measurements
Fruiting Measurements
Discussion
Introduction
Kumquats (Fortunella margarita) were formerly classified with citrus and then were moved to the genus Fortunella, named after Robert Fortune, who introduced kumquats to Europe (Ying, 1998; Rieger, 2006). Kumquats, the smallest edible citrus, are evergreen shrubs or small trees native to southern China but can grow in cooler temperatures (Manner et al., 2006). Unlike typical citrus, the peel of the kumquat is edible and usually sweeter than the pulp (Rieger, 2006; Abobatta, 2018). Kumquats are grown commercially in China and Japan. Four major cultivars given species status by Swingle are as follows: ‘Nagami’ (F. margarita Swingle), ‘Meiwa’ (F. crassifolia Swingle), ‘Hong Kong’ or ‘Hong Kong Wild’ (F. hindsii Swingle), and ‘Marumi’ (F. japonica Swingle) (Abobatta, 2018).
In the subtropical region, large-fruited citrus usually has two flowering periods per year. The first one occurs in late winter or early spring (spring flush), the second in late June (summer flush), and the third in September (autumn flush). Shoots that form in summer and autumn usually have only vegetative growth, while those that form in spring and experience low winter temperatures respond with flowering (Monselise, 1985; Davenport, 1990; García-Luís et al., 1992; Guardiola, 1997). In southern Brazil, citrus flowering occurs when air temperatures decrease to 10°C (Soares-Colletti et al., 2016). Citrus shoots sprout when temperatures are higher than 13°C and sprouting is enhanced by increasing solar radiation (Micheloud et al., 2018). Temperature also affects the development of citrus flowers and fruits. In ‘Comune’ clementine (Citrus clementina Hort. ex Tan.), warmer temperatures (25 or 30°C) during anthesis increase parthenocarpic fruit development (Distefano et al., 2018). Alternate fruit bearing in citrus trees is discussed a lot. In satsuma mandarin (Citrus unshiu) trees, high concentrations of adenosine triphosphate, uridine triphosphate, nicotinamide adenine dinucleotide phosphate, and ascorbic acid were detected in lightly fruiting trees (Nishikawa et al., 2016). Competition for carbohydrates occurs in fruit-bearing ‘Moncada’ mandarin (Citrus clementina) trees, in which the stronger photoassimilate sink of fruit inhibits vegetative growth (Martínez-Alcántara et al., 2015). Regarding carbon utilization, in satsuma mandarin trees, higher concentrations of fructose 6-phosphate, glucose 6-phosphate, and ribulose 1,5-diphosphate accumulated in lightly fruiting trees but more amino acids were found in heavily fruiting trees (Nishikawa et al., 2016). In severely alternate bearing ‘Nadorcott’ mandarin (Citrus reticulata Blanco) trees, the major carbohydrate component of “on” trees was sugars (Ockert et al., 2017) and fruit load in “on” trees inhibited summer vegetative shoot development (Ockert et al., 2018a). Compared to carbohydrates, nitrogen doesn’t seem to be a critical factor in citrus reproductive responses (Ockert et al., 2017, Ockert et al., 2018b).
Similar to large-fruited citrus, small-fruited citrus also have several shoot-sprouting peaks in a year. For example, kumquat and calamondin trees in Japan and Taiwan usually sprout shoots three to five times a year (Iwahori and Tominaga, 1986; Lai and Chen, 2007; Chang et al., 2009). Almost all of the year-round sprouts may bloom, unlike the large-fruited citrus. In Meiwa kumquat (Fortunella crassifolia Swingle), drought stress was positively correlated with flowering (Iwasaki et al., 2000; Ono et al., 2010; Iwasaki and Hiratsuka, 2019); moreover, sugars that accumulate in the xylem play a critical role in osmoregulation (Iwasaki et al., 2019). Since the economic value of small-fruited citrus varieties is far lower than that of the large-fruited citrus, there has been less research on kumquat than on sweet orange or grapefruit (Fadela et al., 2018; Lado et al., 2018; Yilmaz et al., 2018). However, due to the high nutritional value and multi-utility of kumquat and the works we had have done (Chang et al., 2014, Chang et al., 2015), the growth characteristics and cultivation of kumquat need further investigation. Therefore, this study aimed to decipher the phenological stages of the kumquat tree and to establish complete growth behavior data, including shoot sprouting and development, flowering, and fruiting habits. These findings are expected to provide a better strategy for the management of kumquat trees and should benefit the kumquat industry.
Materials and Methods
Plant Material and Experimental Design
Plants were purchased from the Taiwan Provincial Fruit Marketing Cooperative in April 2009. Kumquats [Fortunella margarita (Lour.) Swingle] were grafted on rootstocks of trifoliate orange [Poncirus trifoliata (L.) Raf.]. The seedlings were placed in a greenhouse on the roof of the Department of Horticulture, National Ilan University after purchase. The greenhouse was made from an arched aluminum frame and covered with transparent plastic cloth and measured 12 m long, 7 m wide, and 4 m high. The greenhouse could transmit around 87% photosynthetic photon flux density (PPFD), and there were no additional lighting sources. During the experimental period, the mean outdoor light intensity changes in summer and winter were 1100 ± 130 and 780 ± 63 µmol·m-2·s-1, respectively. The greenhouse was surrounded by anti-insect nets, and fans were placed at the four corners to maintain microclimate homogeneity and avoid high- temperature retention during the summer.
The plants were transferred to circular plastic pots with a diameter of 25 cm and a volume of 8 L in January 2010. The filling medium was field soil:sand:peat:coconut fibers in a volume ratio of 2:4:1:1. The field water capacity and wilting point of the mixed medium were 40% and 25% (wt/wt), respectively. During the experimental period, the mixed medium in the pots was maintained at the field water capacity and moisture management was carried out according to weather changes. The pots were watered 1 to 2 times each day during high temperatures in summer and once every 2 to 3 days during low temperatures in winter. Fast-acting liquid fertilizer (Peter’s N:P:K = 20:20:20) was applied once every two weeks by diluting a 1,000 mL solution 1,000 times. Every three months, 35 g of slow-acting fertilizer (Hi-Control No. 1, 180-day, N:P:K = 14:12:15) and 30 g of organic fertilizer (Jinyu Fertilizer, N:P:K:organic matter = 4:1:1:77) was applied. The recommendations in the Plant Protection Manual were used as a reference for pest and disease control and implemented based on actual needs.
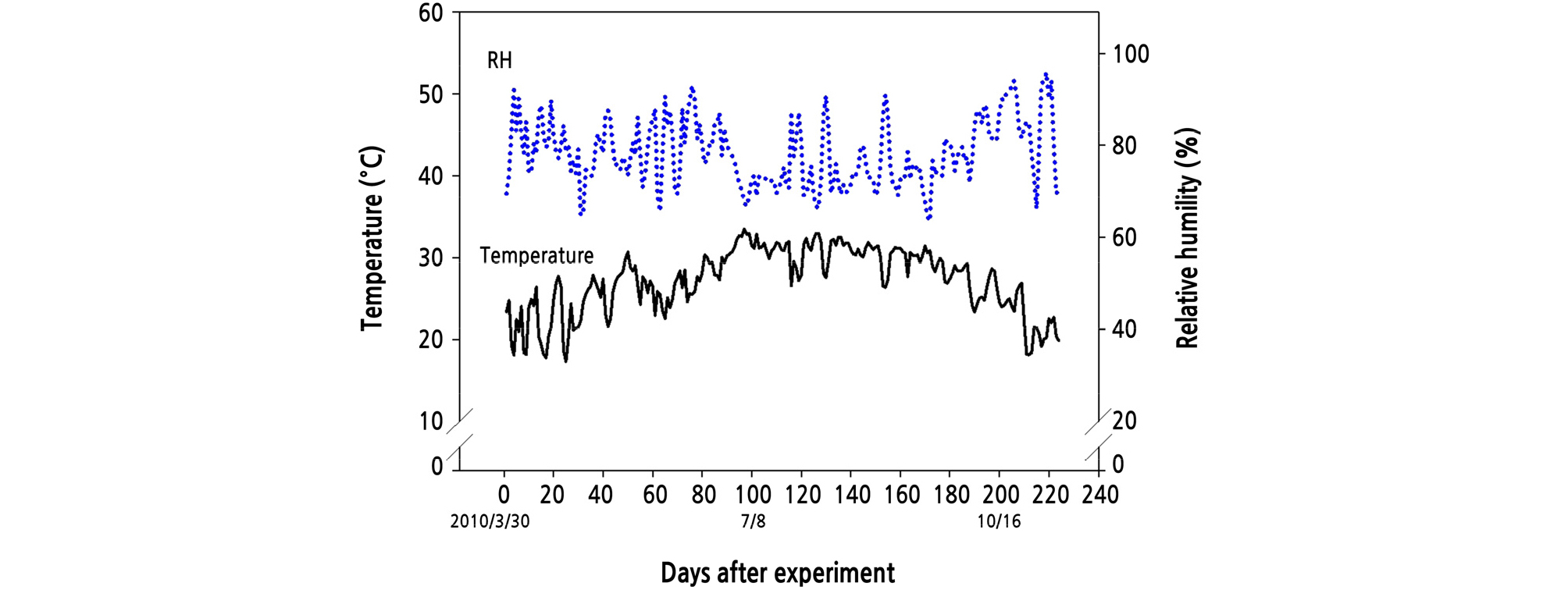
On March 18, 2010, plants with similar rates of new shoot sprouting were used for the experiments. The air temperature changes in the greenhouse were recorded using a temperature and humidity recorder (EL-USB-2-RH/Temperature Data Logger, Lascar Electronics Ltd., UK). Air temperature changes were recorded every hour. In order to simplify the data, the 24 air temperature data points that were recorded in 1 day were averaged and converted to average daily temperature. Fig. 1 shows the average daily temperature changes for the greenhouse during the experimental period.
Phenological Stages of Kumquat
In 2010 ‑ 2011, kumquat phenology was recorded and described based on the BBCH scale for the main phenological stages proposed by Lancashire et al. (1991). Forty healthy plants with similar growth performance in the greenhouse were selected for phenological observation. Phenology was recorded once every 1 ‑ 2 weeks based on the changes in plant morphology, and a digital camera (Nikon D80, Japan) was used to photograph the plants.
Shoot Growth Pattern and Measurements
Six kumquat plants with similar growth performance inside the greenhouse were selected for measurement of annual growth. Ten new sprouting buds (first flush) were selected from every tree to be used as shoots for continuous recording. As kumquats have multiple flushes in one year, the shoots from different flushes were recorded and analyzed individually in order to elucidate the growth characteristics of shoots from different flushes. The flush sequence was defined as the sequence in which buds sprout in the first year. The first flush was shoots sprouted in spring from the branches of the previous year, and the shoots that sprouted from the first flush were defined as the second flush, and so on (Chang et al., 2014). The sprouting time of different flush sequences was defined as a new shoot sprouted for more than 50% of the same flush sequence in a plant. The experimental recording period was from March 2010 to December 2011. The number of newly sprouted buds, shoot length, and shoot diameter was recorded once every two weeks.
The mean relative growth rate (RGR) for shoots was calculated based on the method of Hunt (1990) as follows:
| $$RGR\;of\;shoot\;\leq\;ngth=\frac{\ln(L_2)-\ln(L_1)}{t_2-t_1}$$ | (1) |
| $$RGR\;of\;shoot\;diameter=\frac{\ln(D_2)-\ln(D_1)}{t_2-t_1}$$ | (2) |
Where L2, L1, D2, and D1 are the shoot lengths and the diameters of specific shoots at continuous time points of t2 and t1, and ln is the natural logarithm.
Flowering and Fruiting Measurements
Plants used for shoot growth measurements were also used for recording flowering and fruiting characteristics. The phenological changes in reproductive buds in the selected plants were recorded from March 2010 to December 2011. The recording was carried out once every 1 ‑ 2 weeks based on the speed of phenological changes during the flowering and fruiting period. The number of flowers on the shoots was the mean number of flowers that bloomed on the recorded shoots while the ratio of the number of flowering nodes to the total number of nodes in each shoot was defined as the shoot flowering rate. The mean number of fruit of the different shoots is taken to be the number of fruit per shoot. The fruit lengths and fruit diameters were measured once every week.
Statistical Analysis
Calculations of data and plots were performed by using SigmaPlot 10.0 (SPSS Inc., Chicago, IL, USA) program package. Equations expressing the changes in shoot length and the number of flowers, days after anthesis, and fruit size were derived from non-linear regression analysis by using the same program package. Each value represents the mean ± SE (n = 6 trees).
Results
Phenological Stages of Kumquat
The description of the stages, according to the BBCH codes, is as follows:
11: First leaves sprouting. First leaves observed and unfold, the axis of developing shoots visible (Fig. 2A).
15: More leaves unfold. More leaves unfold but have not reached the full size; meanwhile, the axis of shoots increases (Fig. 2B).
19: Leaves fully develop. Leaves fully expand (Fig. 2C).
51: Appearance of flower buds. Buds are visible and closed, shoot elongation terminates (Fig. 2D).
55: Flower buds visible. Flower buds swell but are still closed (Fig. 2E).
60: First flower opens (Fig. 2F).
65: 50% of the flowers open. At least 50% of flowers open and the first petals are falling (Fig. 2G).
68: Petal fall. Flowers fell, and petals collapse (Fig. 2H).
71: Fruit setting. Ovary grows and fruit has a longitudinal diameter of nearly 5 mm (Fig. 2I).
78: Fruit growth (80% of final size). Fruit reaches 80% of full size (Fig. 2J).
89: Fruit ripe for picking. Fruit reaches the full size and well colored for harvest (Fig. 2K).

Fig. 2.
Phenological growth stages of kumquat [Fortunella margarita (Lour.) Swingle] trees. A: 11: Visible leaves unfolded and started spreading. B: 15: More leaves unfolded but had not yet reached the full size. C: 19: Leaves fully developed. D: 51: Flower buds appeared. E: 55: Flower buds kept growing, but buds remained closed. F: 60: First flower opened. G: 65: 50% of the flowers opened. H: 68: Most petals dried and fell. I: 69: End of flowering, fruit set was visible. J: 78: Fruit reached 80% of the final size. K: 89: Fruit was fully ripe for commercial picking.
Shoot Growth Pattern and Measurements
The number of buds sprouted exhibited significant variations among different flush sequences (Fig. 3). The shoots of the second, third, and fourth flush sprouted in mid-May, early July, and early September, respectively, about 70 ‑ 80, 112 ‑ 122, and 168 ‑ 178 days after the shoots of the first flush sprouted. Moreover, the duration from new buds of the second flush sprouting to those of the third flush sprouting was 56 days, while the duration between the third flush sprouting to the fourth flush sprouting was also nearly 56 days. The maximum average number of new buds sprouted from each first flush shoot was approximately 5 for the second flush, 2 for the third flush, and 0.4 for the fourth flush.
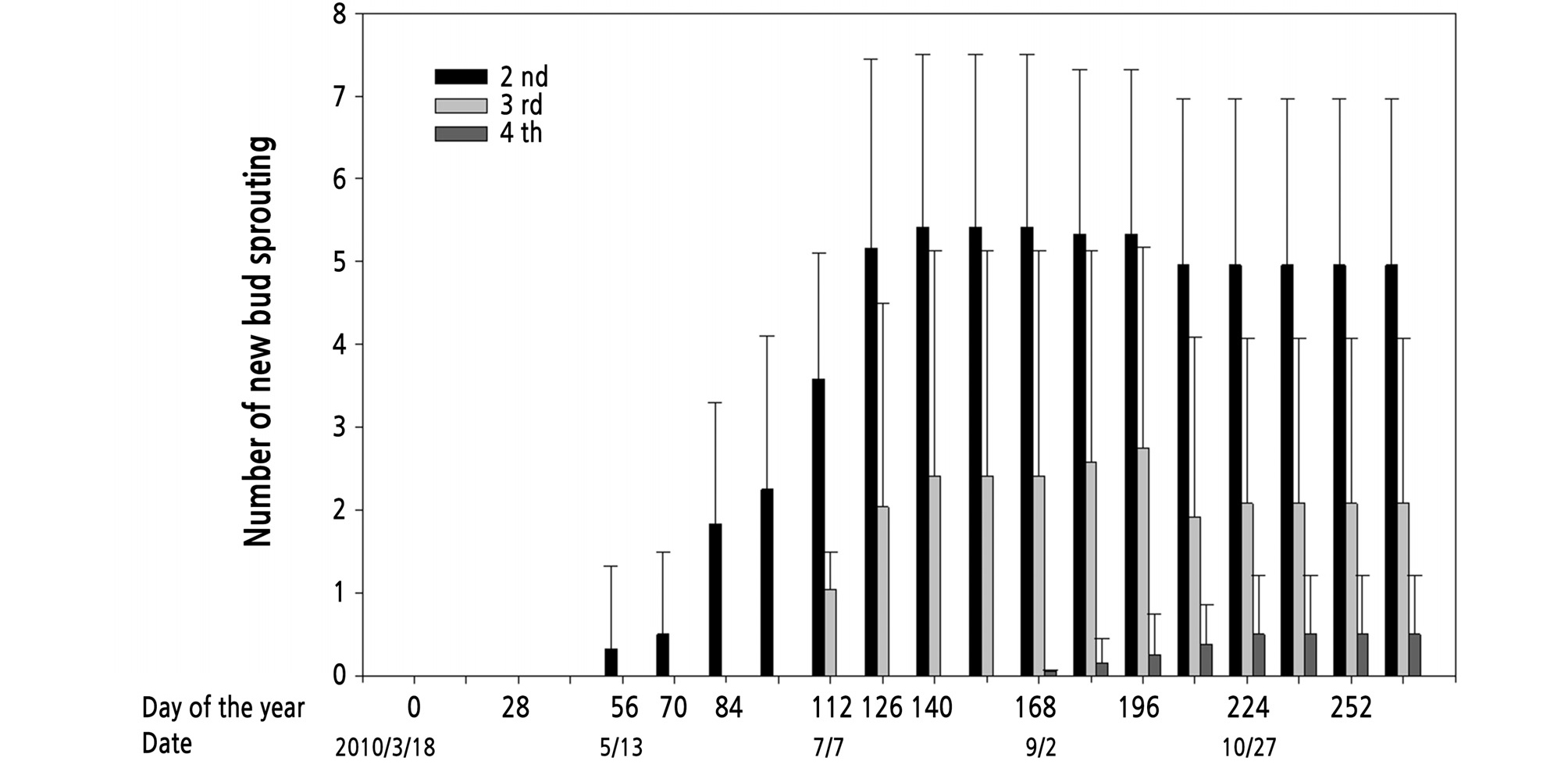
Fig. 3.
New sprouting buds in flushes of different sequences in kumquat [Fortunella margarita (Lour.) Swingle] trees. The average was counted by numbers of buds sprouted per first flush shoot in 2010. Each value represents the mean ± SE (n = 6 trees). The vertical bars represent standard errors of the averages.
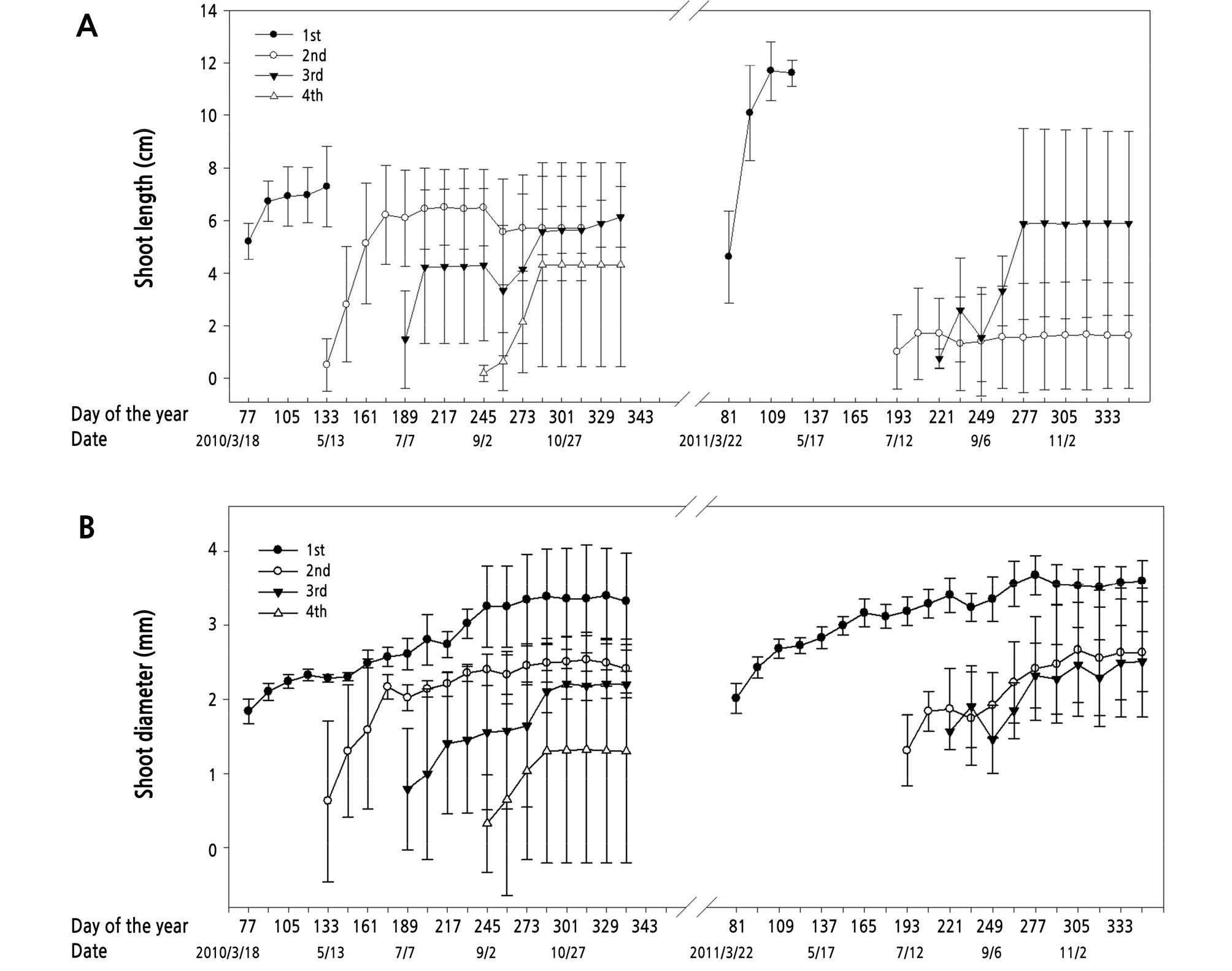
Fig. 4A illustrates the variance of shoot length of kumquat trees from 2010 to 2011. In 2010, the termination of shoot elongation for the second, third, and forth flushes was after four, two, and four weeks of bud sprouting, respectively. The average shoot length among the flush sequences was longest in the first flush and shortest in the fourth one. In 2011, the new buds of the first flush sprouted in mid-March, while the new buds of the second flush sprouted until mid-July, followed by the sprouting of the third flush buds in early September. Similar to the previous year’s results, the average shoot length was the greatest in the shoots of the first flush, which was approximately two times longer than the shoots of the second flush.
Unlike the variances of shoot length, which exhibited distinct shoot elongation termination, shoot diameter showed an increasing trend during the whole experimental period. The average shoot diameter was the largest in the shoots of the first flush and showed a declining trend with the flush sequence increasing, with the fourth flush having the smallest shoot diameter among all of the flush sequences (Fig. 4B).
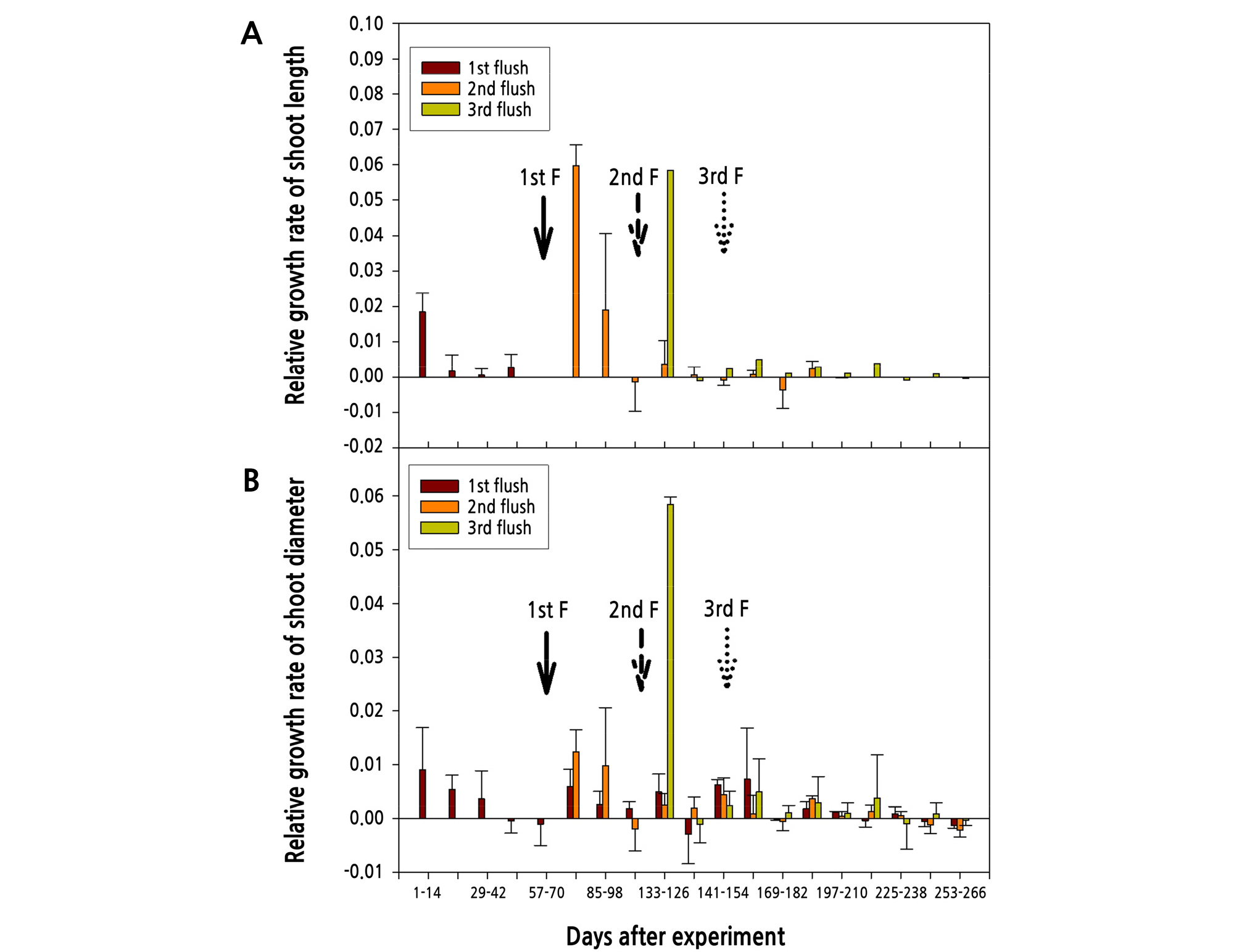
Once the growth data of shoot lengths and diameters were converted into relative growth rates (RGR), the intensities of shoot vegetative growth exhibited relationships to the flowering potential of shoots (Fig. 5). Regardless of the flush sequence, variances of the shoot length generally exhibited a higher growth peak at the initial stage after shoot sprouting and shoot growth nearly ceased a few weeks later. Therefore, the shoot expressed elongation termination significantly, moreover, came after the appearance of flowering (Fig. 5A).
Flowering Measurements
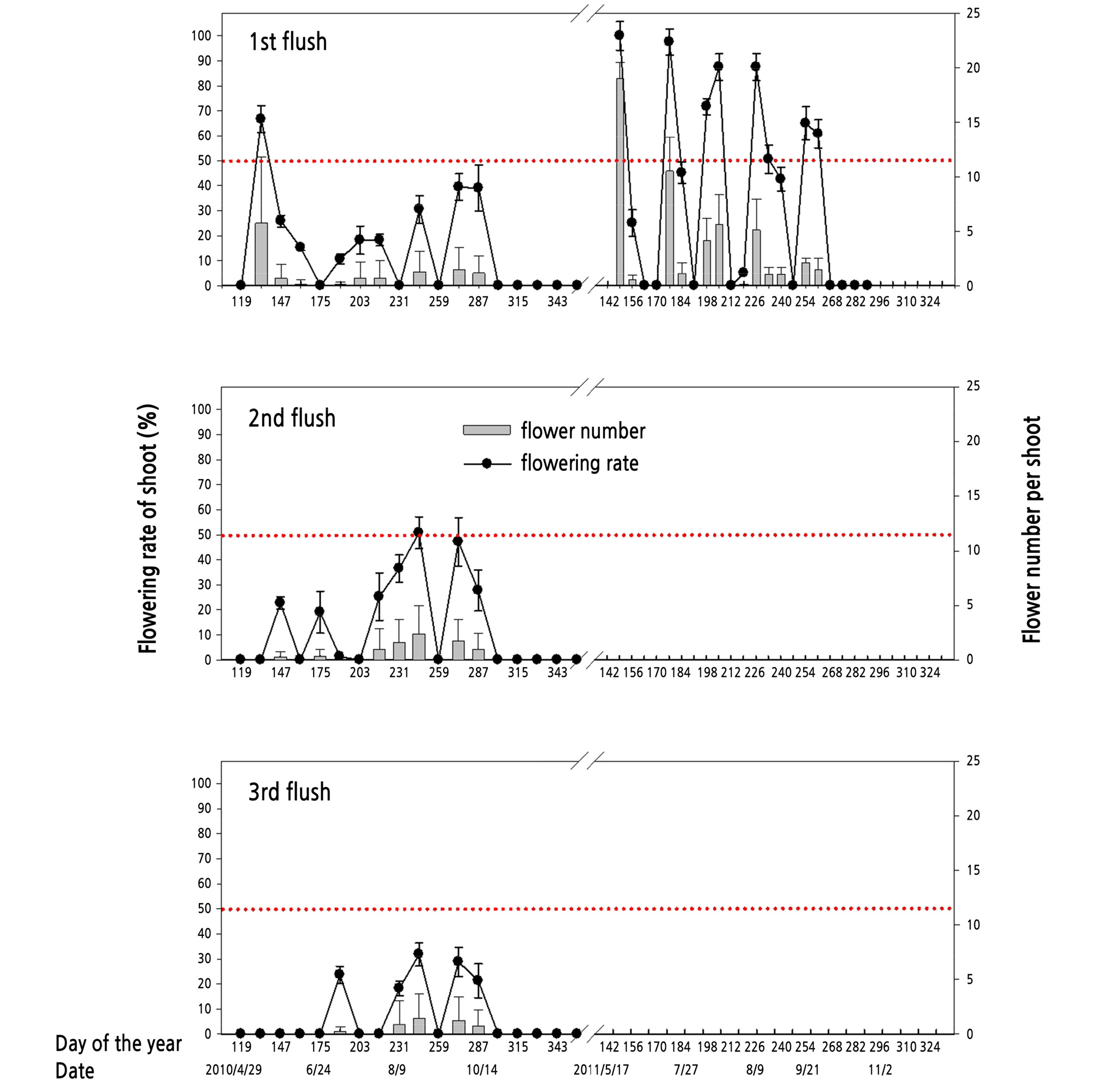
Fig. 6 shows the pattern of repeated flowering in kumquat. The flowering rate of the shoot and flower number per shoot decreased as flush sequences increased. From 2010 to 2011, the shoots of the first flush expressed higher flowering potential than those of the second or third flushes. The shoots of the first flush had four flowering peaks in 2010 and five peaks in 2011. Both the flowering rate and number were higher in 2011 than those in 2010. In 2010, only the first flowering peak reached the flowering rate of nearly 70%, while the flowering rates of other peaks were lower than 50%. In 2010, the flowering performance of the shoots of the second flush was inferior to that of the first flush, and the flowering performance of the third flush shoots was even lower. In other words, the later the shoot was sprouting, the lower the flowering ability of the shoot. However, in 2011, after the flowering on the shoot of the first flush, there were no more flowering peaks recorded in the shoots of the second or third flush because the fragmentary flowering observation was inferior to analysis.
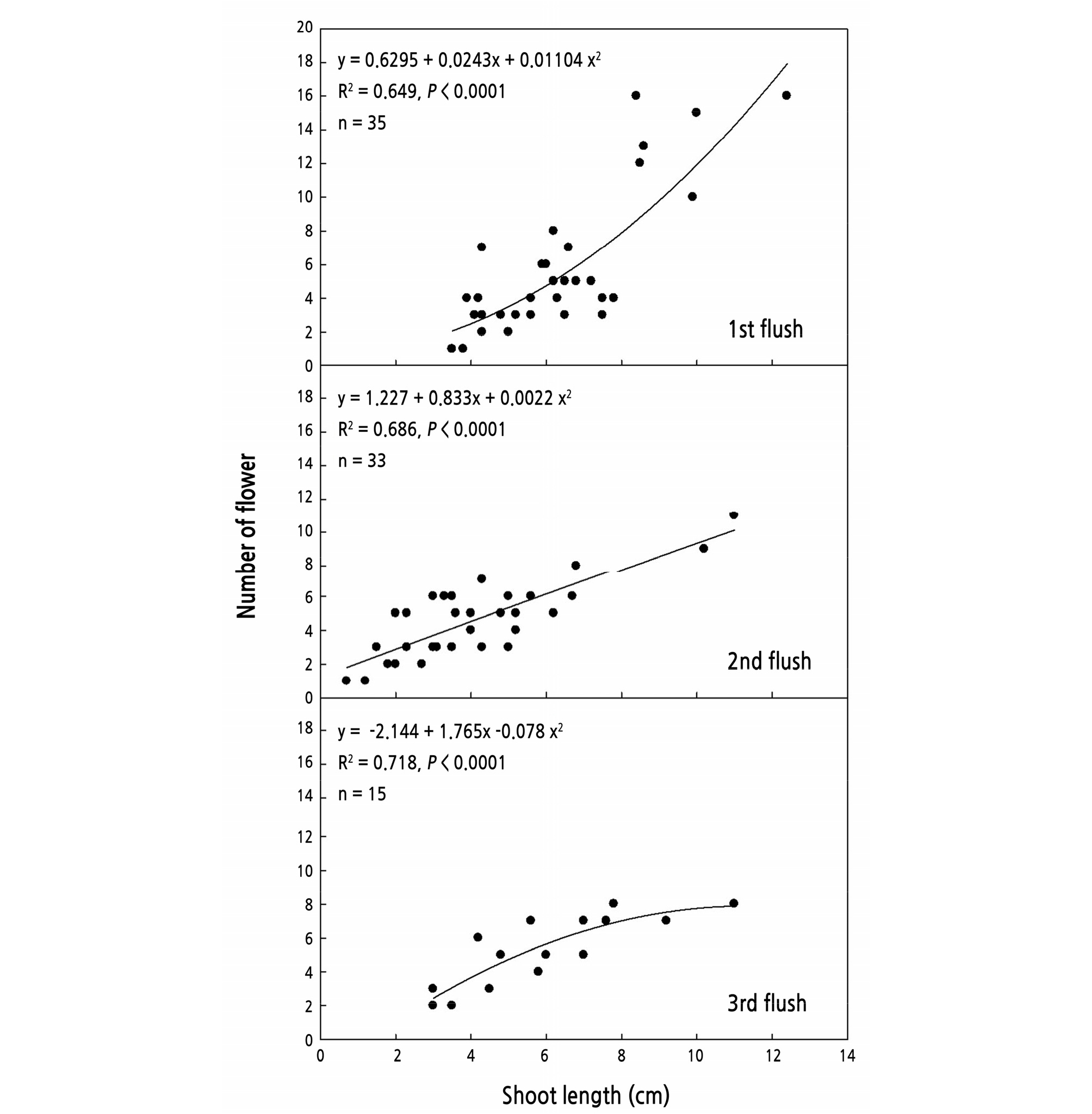
The number of flowers on the shoot was relative to the length of the shoot (Fig. 7). In the results of regression analysis between shoot lengths and flower numbers, the R2 were 0.649, 0.686, and 0.718 of the first, second, and third flushes, respectively. In other words, increasing the length of shoot exhibited positive effects on flower numbers.
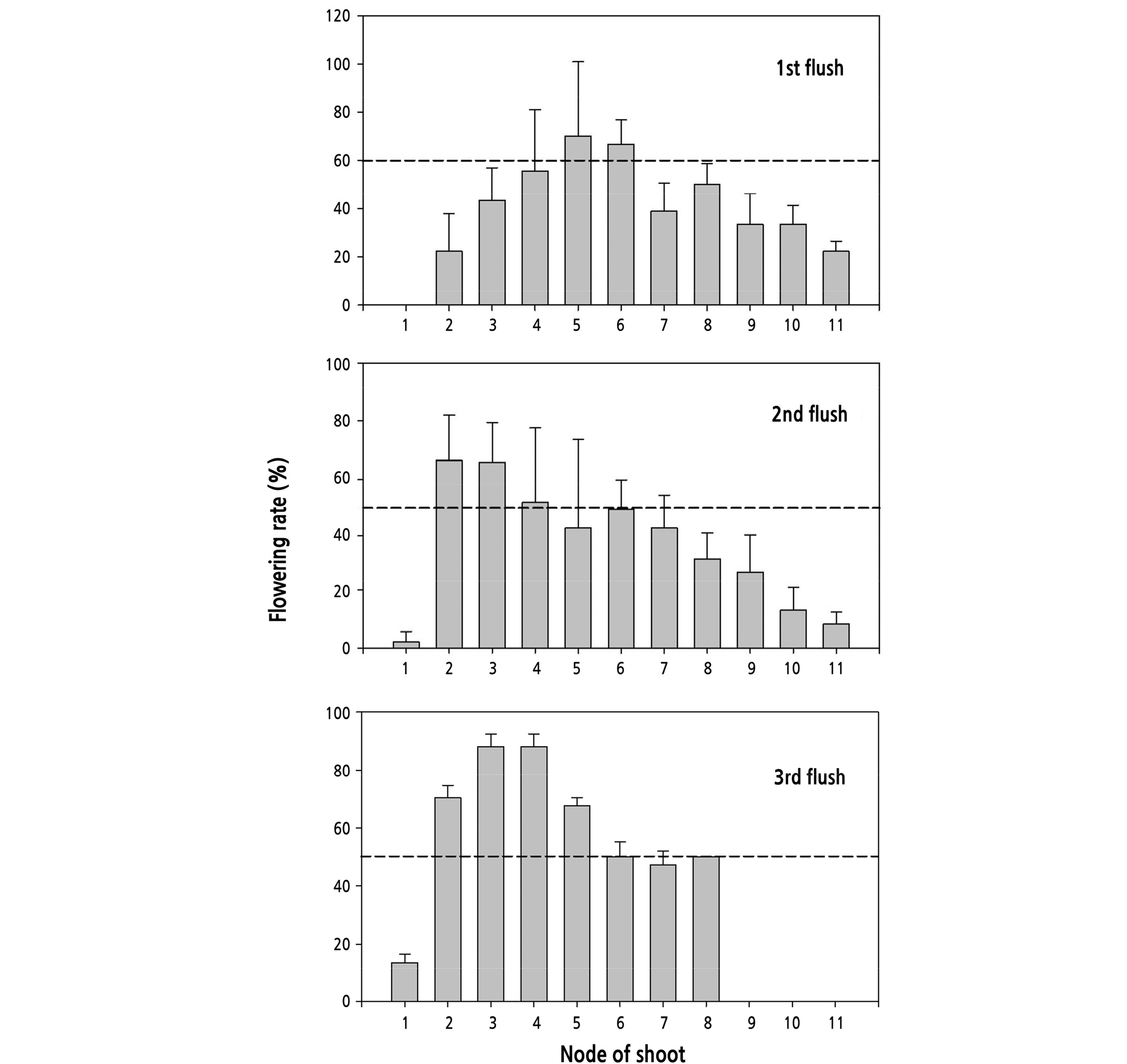
The node position was counted along with the shoot and the node in the axis position nearest the apex was considered as Position 1, the node in the nearest leaf axis following this first position was considered to be Position 2, and so on. The higher flowering rates of the first flush shoots were at nodes located in the middle shoot segment, in which flowering rates of node positions 4, 5, and 6 exceeded 50%. The node flowering rates of the second and third flushes were slightly different from the first one since they expressed higher flowering intensities at nodes located in upper shoot segments from position 2 to 4 (Fig. 8).
Fruiting Measurements
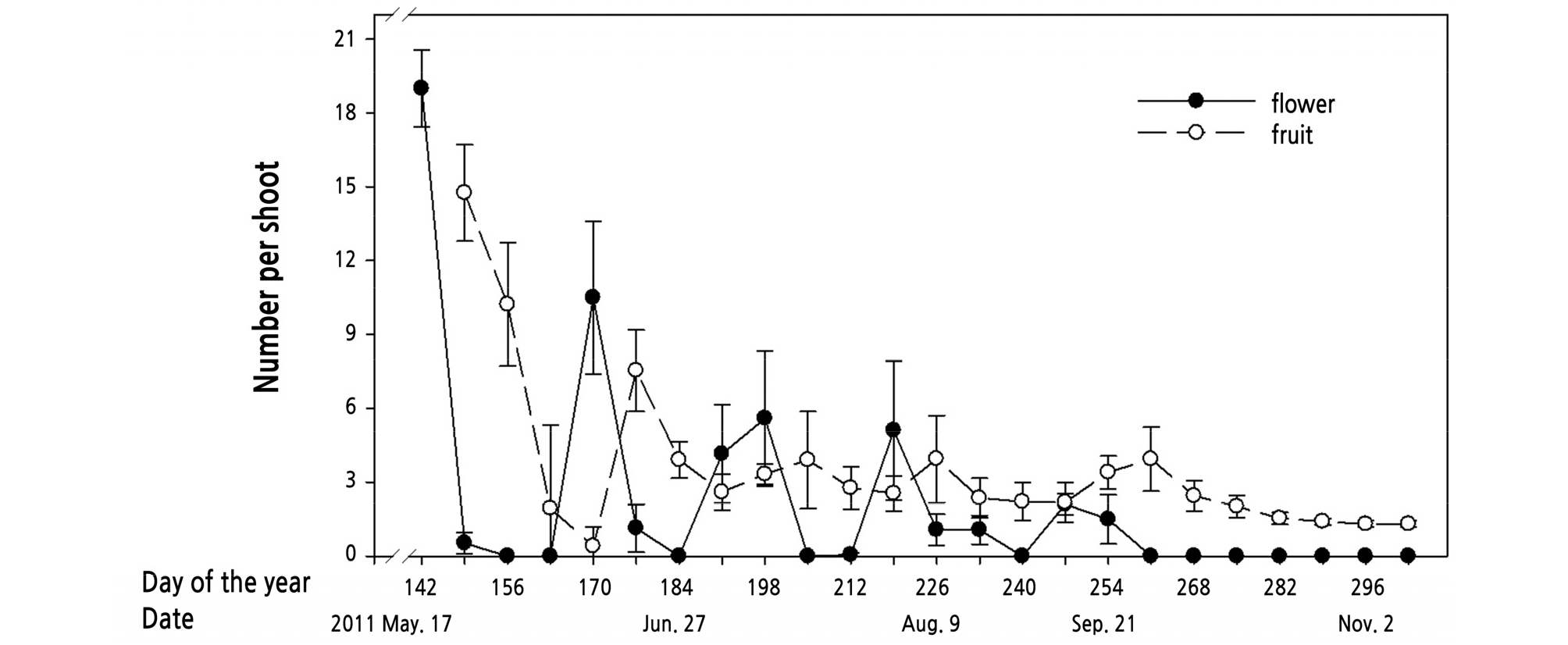
The first peak of fruit number occurred in late May, one week after the flowering peak, with nearly 15 fruitlets on every shoot. However, the fruitlet number decreased rapidly, and only a few fruitlets remained three weeks later. Fruitlets still occurred after the following flowering sequences; however, the number of fruitlets declined noticeably (Fig. 9).
The initial fruitlet number was highest in the first flowering peak (I) followed by the second flowering peak (II). However, most fruitlets of the first flowering peak (I) could not be found at the end of the experiment, while a few fruits formed from flowers of the second flowering sequence remained. Fruitlets from flowers of the third (III), fourth (IV), and fifth (V) flowering sequence were not only far fewer than those of flowering peaks I and II but also exhibited poor fruiting potential, of which almost none could be harvested in the final stage of the experiment (Fig. 10).
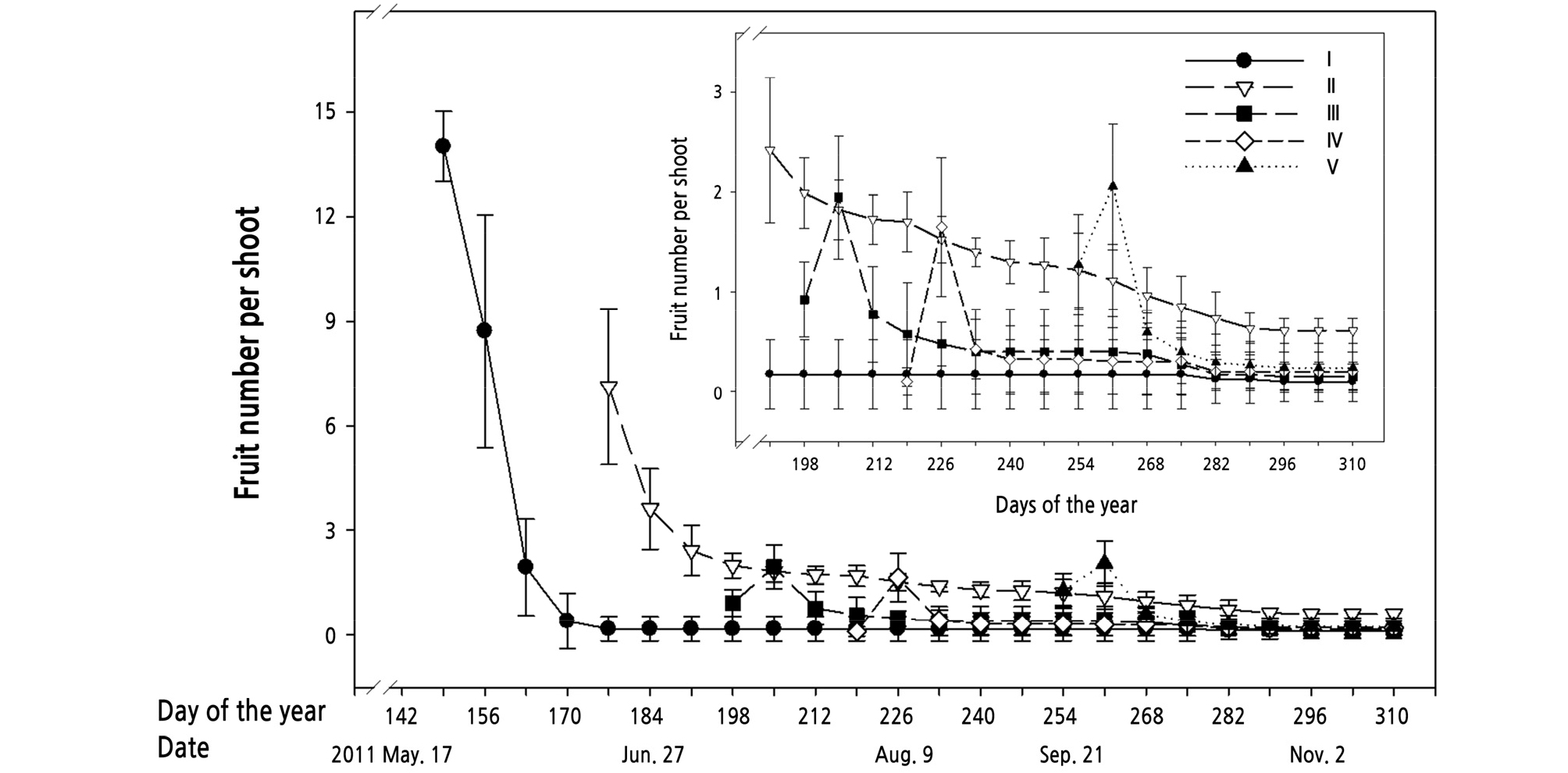
Fig. 10.
Fruit numbers in the different flowering sequences of the first flush of kumquat [Fortunella margarita (Lour.) Swingle] trees. “I” indicate the first sequence flowering of the first flush shoot, and “II” means the second sequence flowering of the first flush shoot, and so on. Each value represents the mean ± SE (n = 6 trees). The vertical bars represent standard errors of the averages.
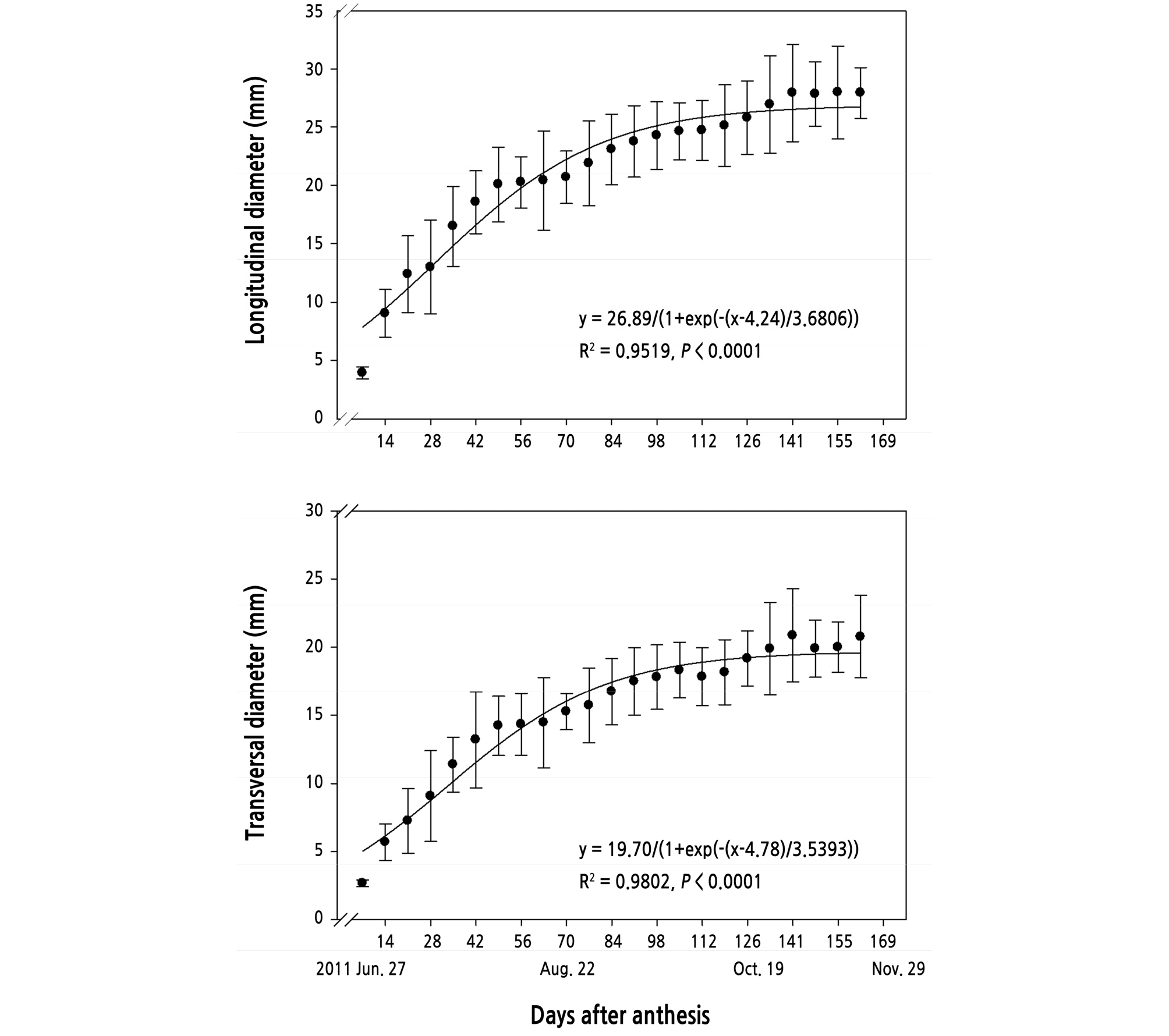
The longitudinal and transversal diameters of kumquat fruits increased with increasing days after bloom, and the final fruit size averaged 28 mm in a longitudinal section and 20 mm in transversal one (Fig. 11).
Discussion
The kumquat displayed multiple sprouting flushes and repeatedly bloomed during a whole year. Trees sprouted four flushes in 2010, whereas only three flushes appeared in 2011. The decrease in bud sprouting might be caused by continuing fruiting, which delayed new bud sprouting and increased tree nutrient consumption. The first, second, third, and forth flushes sprouted in mid-February to March, mid-May, early July, and early September, respectively. The number of buds sprouting and shoot extension duration was inversely proportional to flush sequences (Fig. 4). The shoots that sprouted in the early season, February to May, exhibited higher bud sprouting potential and the larger shoot size than those that sprouted in the late season, July to September.
In tropical climates, new buds may sprout throughout the year (Monselise, 1985; Spiegel-Roy and Goldschmidt, 1996), whereas in subtropical regions, bud sprouting occurs two times per year (Monselise, 1985; Davenport, 1990). The bud spouting behavior of citrus is similar to that of kumquats. Taiwan’s summer or early autumn temperatures are similar to those of tropical climates, and temperatures generally exceed 32°C. In such high temperatures, the bud sprouting of kumquats is continuous and numerous. In environments with an average temperature of 22°C and 28°C in spring, the amount and frequency of bud sprouting in kumquat were significantly less. However, the more frequent the shoot sprouting, the shorter the shoot growth duration was. The shoots formed at higher temperatures were smaller than those formed at lower temperatures. The shoots of the first and second flushes that sprouted between February and May had sufficient growth periods to allow the growth and development of flowers and fruit.
The flowering habit of the kumquat is similar to that of most citrus, in which flower buds are produced after shoot elongation stops (Chang et al., 2009). However, kumquat differs from the shoots of large-fruit citrus, in which shoots formed in high temperatures (summer and autumn) are always in vegetative growth (Moss, 1969; Monselise 1985; Davenport, 1990; García-Luís et al., 1992; Guardiola, 1997; Soares-Colletti et al., 2016; Micheloud et al., 2018), whereas flowering of kumquats occurred in shoots formed both in low temperatures (spring) and high temperatures (summer and autumn) (Fig. 6). The capability of flowering is always apparently influenced by the maturation level of shoots. After experiencing low temperatures in winter, large-fruit citrus usually blooms in the spring and forms flower buds on the shoots that sprouted last summer (Monselise, 1985; Davenport, 1990; Soares-Colletti et al., 2016; Micheloud et al., 2018). In Taiwan, kumquats sprouted the first and second flushes from spring to early summer, when the average temperatures rarely exceeded 28°C, which offered more extended periods for shoot expansion and carbohydrate accumulation. The shoots of the third and fourth flushes usually sprouted from July to September, when the average temperature exceeded 32°C. The high temperature made the shoots prematurely mature, and the shoot length and flowering potential decreased significantly.
Fruit set occurred after almost seven days of flowering in kumquats (Fig. 9). The number of fruit on the tree decreased rapidly in the first weeks, then remained lower than that of the initial fruitlet stage. In citrus, the percentage of flowers that set and develop into a mature fruit is low, ranging from 0.1 to 0.3% (Guardiola, 1997). Furthermore, even though flower formation is an essential factor for fruiting; the percentage of fruit set is inverse to that of flower number (Guardiola, 1997). The complicated relationship between flowering and fruiting is also observed in kumquat trees. The repeated bud sprouting habit caused abundant flowering of shoots while the fruit set percentage was lower than 0.3%. This indicates that extensive flowering does not promote more fruiting, and most of the flowers do not form fruit. Shoot maturation levels influence flowering as well as fruiting. In 2011, high fruiting potential only occurred on the first flush shoots, sprouted in March. The shoots sprouted after July seldom had a successful fruit set. The fruit’s size and quality of ‘Meiwa’ kumquat are the largest when they develope from the first flowers (Iwahori and Tominaga, 1986), which display similar growth habits as kumquat.
Moreover, the different flowering sequences in the same flush also exhibited variations in fruiting potential. The initial fruitlet number decreased as the sequence of flowering peak increased. Therefore, the highest fruitlet number occurred in the first flowering peak (I), followed by the second (II), and so on. Furthermore, only the fruit formed by flowers of the second flowering peak developed into mature fruit. The results indicated that the temperature might influence the kumquat fruit set. Fruit that set after mid-July (the third (III), fourth (IV), and fifth (V) flowering sequence) had limited development and did not mature. High temperatures might be harmful to fruit development in the initial enlargement stage and caused lots of fruit falling.