Introduction
Materials and Methods
Sampling
GC-MS Analysis
RNA Isolation and cDNA Synthesis
qRT-PCR
Results
Analysis of Floral Volatile Compounds
Expression Analysis of Floral Scent Genes
Discussion
Introduction
Maxillaria are neotropical orchids with approximately 400 species. They originated in Central America and have many colorful flowers that bloom during the late winter for 2 months (Dathe and Dietrich, 2006; Davies et al., 2005). M. tenuifolia, or Lindl., is called the “coconut orchid” due to its consistently strong coconut-like scent that is responsible for pollinator attraction and was recognized as the best scented orchid at the 18th World Orchid Conference (Flach et al., 2004; Perraudin et al., 2006). Flowers emit various types of floral scents to attract potential pollinators (Muhlemann et al., 2014). In addition, floral scent is an important trait in the flower industry. Recently, the domestic orchid industry has been declining because of its dependence on exports and the current market saturation. Therefore, it is necessary to find optimal ways to increase profits, such as by increasing demand for orchids by introducing a special orchid that has a novel scent. In this sense, M. tenuifolia is an orchid with a unique floral scent and shape. The breeding of Cymbidium and Phalaenopsis orchids, which are the main orchids in the industry, has been studied; however, fragrance research is lacking. With the availability of technological advances, fragrance product lines, such as cosmetics, perfumes, and food, have grown because of the effect of volatile compounds on humans (Baek et al., 2016). M. tenuifolia, in this respect, is increasingly cultivated because more consumers choose it for its floral scent.
Terpenes are diverse chemical groups that are produced by plants and are representative compounds of scent. Terpenes include monoterpenes, such as linalool, limonene, and myrcene, and sesquiterpenes, such as caryophyllene, farnesene, and copaene (Cseke et al., 1998; Dudareva et al., 2000; Fu et al., 2019; Maiti and Mitra, 2019). Terpenoids are significant compounds with potential therapeutic effects on human and animal diseases (Turkez et al., 2014a). The volatile compounds of M. tenuifolia were demonstrated to include α-copaene, β-caryophyllene, δ-decalactone (known as a coconut scent), 1,8-cineole, limonene, β-myrcene, α-pinene, β-pinene, and sabinene (Gerlach and Schill, 1991; Perraudin et al., 2006). Most Maxillaria fragrance studies only report the chemical composition of the floral scent, and there are no data on the floral organs and flowering stage. Headspace–solid-phase microextraction–gas chromatography–mass spectrometry (HS-SPME-GC-MS) is primarily used for the analysis of volatile compounds. This method can be used to quantitatively and qualitatively analyze solid samples directly. Terpenoids are the major volatile compounds in many flowers including orchids. Linalool, myrcene, nerol, and 2-hexanol were detected in Phalaenopsis bellina using gas chromatography-mass spectrometry (Hsiao et al., 2006). Terpenoids are synthesized through two different pathways, the mevalonic acid (MVA) pathway in the cytoplasm and the methylerythritol phosphate (MEP) pathway in the plastids, which are responsible for sesquiterpenes and monoterpenes, respectively (Ramya et al., 2017). In quantitative real timePCR (qRT-PCR), a target cDNA is amplified for quantification of gene expression both to target and reference genes with a constant volume of crude cDNA, which improves the sensitivity and specificity (Guenin et al., 2009). Using these systems, M. tenuifolia were analyzed to profile the fragrance of floral parts at various flower developmental stages. This research produced baseline data for scent analysis of M. tenuifolia and for the breeding of orchids for aromatics to promote the floricultural industry.
Materials and Methods
M. tenuifolia was obtained from the Lee Won Orchid Nursery (Gimpo, Korea) and cultivated in the experimental greenhouse of the National Institute of Horticultural and Herbal Science (Wanju, Korea). The experiment was conducted between April and May. Samples were prepared in triplicate.
Sampling
The flowers were sampled at 13:00 on a clear day, at 200 ± 6 µmol·m-2·s-1 and at a temperature of 25 ± 2°C and relative humidity of 20% ± 3%. The floral organs were separated into petals, sepals, lip, and column. The flowering stages for GC-MS analysis were the closed flower bud (I), the initial flowering stage (II), the full flowering stage (III), the loss of pedicel color stage (IV), and the wilting flower (V) (Kim et al., 2016). Each sample was immediately sealed in a 20 mL tube, and stages I, II, and III were frozen in liquid nitrogen for RNA isolation.
GC-MS Analysis
An SPME device with a 100-µm polydimethylsiloxane (PDMS) fiber was used for the volatile extraction. The floral volatiles were analyzed using HS-SPME-GC-MS on a 7000C GC-MS system (Agilent Technologies, Wilmington, DE, USA). The GC was equipped with a DB-5MS column (30 m × 0.25 mm I.D., 0.25 µm, Agilent Technologies, Wilmington, DE, USA). The temperature was held at 60°C for 5 min and then raised to 250°C at 3°C/min. The injector and detector temperature was maintained at 250°C. The carrier gas helium flow rate was 1.0 mL/min. The MS detector was used in the EI mode with an electron energy of 70 eV, and the data were fully scanned at a rate of 1 scan/s over the m/z range of 30 - 350 amu. The transfer line was at 280°C. The identification of the HS-SPME-GC-MS data was performed by comparison with either n-alkane or the NIST 13 (National Institute of Standards and Technology, Gaithersburg, MD, USA) mass spectral library, and retention indices (RI) of the compounds were determined using the Kovat index. The GC-MS values of the volatile compounds are shown as the means ± SD of triplicates. The SPPS program (SPPS Inc., Chicago, IL, USA) was used to determine the distribution of the volatile constituents. In addition, Duncan’s multiple range test was performed to check the volatile emission changes of the main compounds in a daily cycle.
RNA Isolation and cDNA Synthesis
M. tenuifolia samples were ground into a powder with a mortar and pestle with liquid nitrogen, and the RNA was isolated using the RNeasy Plant Mini Kit (Qiagen, Germany) according to the manufacturer’s protocol. The quality and quantity of RNA were verified using the QuickDrop spectrophotometer (Molecular Devices, USA). First, strand cDNA was synthesized from the total RNA using the PrimeScriptTM 1st strand cDNA synthesis kit (Takara, Japan) following the manufacturer’s instructions. A total of 200 ng of RNA was used for reverse transcription with dT18 primers, and 1 µL of this reverse transcription product was diluted in 20 µL of ddH2O for a template using the Primescript RT reagent kit with a gDNA eraser (TaKaRa). The cDNA products were loaded into a 1% agarose gel electrophoresis in 10× TBE buffer. Gels were visualized by Loading STAR (Dyne Bio, South Korea), and the images were documented using the GelDoc-It Imaging System (Analytik Jena, UK).
qRT-PCR
For q-RT-PCR analysis, we designed primers according to the volatile compounds, and some terpenoid genes were selected. All primers for qRT-PCR are listed in Table 1. The ubiquitin-containing actin gene was used as an internal control. The cDNA diluted to 200 ng·µL-1 was used for the qRT-PCR analysis. Rotor-Gene SYBR Green PCR Master Mix (Qiagen, Germany) was used with 1 µL of template for a total reaction volume of 20 µL. For the analysis of gene expression, we obtained three biological and technical replicates for each gene using the Rotor-Gene 6000 real-time rotary analysis system (Qiagen, Germany). The amplification stage was for 10 min at 95°C, followed by 40 cycles of 30 s at 95°C, 30 s at 59°C, and 40 s at 70°C. Values are provided as the means ± SD.
Table 1. Primers used for qRT-PCR analysis
Results
Analysis of Floral Volatile Compounds
The volatile compounds were classified accordingto chemical structure and origin of synthesis, as monoterpene, sesquiterpene, and aliphatic (Table 2). The total volatile compound amount of each floral part is as follows: petal 94.00%, sepal 93.02%, lip 72.71%, and column 89.99%. In the petal, two monoterpenes, eight sesquiterpenes, and two aliphatic compounds were identified, and the most abundant compounds were β-caryophyllene (66.28% ± 5.40%), α-copaene (11.39% ± 3.12%), caryophylladienol Ⅱ (8.72% ± 0.32%), and δ-decalactone (3.59% ± 2.35%). In the sepal, two monoterpenes, seven sesquiterpenes, and two aliphatic compounds were identified including β-caryophyllene (65.50% ± 3.86%), α-copaene (12.85% ± 2.53%), aryophylladienol Ⅱ (7.19% ± 0.72%), and caryophellene oxide (2.02% ± 0.16%). In the lip, two monoterpenes, seven sesquiterpenes, and one aliphatic compound were identified including β-caryophyllene (68.13% ± 1.19%), α-copaene (9.16% ± 2.42%), caryophylladienol Ⅱ (8.43% ± 0.27%), and 4,8,8-trimethyl-2-methylene-4-vinylbicyclo [5.2.0]nonane (1.5% ± 0.26%). In the column, 3 monoterpenes, 10 sesquiterpenes, and 2 aliphatic compounds were identified, and the most abundant compounds were β-caryophyllene (49.51% ± 0.00%), α-copaene (29.58% ± 0.00%), caryophylladienol Ⅱ (5.09% ± 0.00%), and the same amount of δ-cadinene and α-patchoulene (1.63% ± 0.00%).
Table 2. Percentage of volatile compounds identified in M. tenuifolia floral organs using HS-SPME-GC-MS
yRelative contents (%) = (area under peak/total peak area) × 100.
xAll data are presented as means ± SD (n = 3).
The volatile compounds of each stage were grouped into monoterpenoid, sesquiterpenoid, and aliphatic (Table 3). The total volatile compound amount of each flowering stage was as follows: the bud stage (Ⅰ) 29.07%, the initial flowering stage (Ⅱ) 80.96%, the full flowering stage (Ⅳ) 93.67%, the loss of pedicel color stage (Ⅴ) 85.56%, and the wilting flower stage (Ⅵ) 69.75%. In the bud stage (Ⅰ), nine aliphatic compounds were identified; among them, l-(+)-ascorbic acid 2,6-dihexadecanoate (11.47% ± 4.06%) and 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione (10.57% ± 2.84%) were detected the most. In the initial flowering stage (Ⅱ), seven sesquiterpenes and one aliphatic compound were identified, and the most abundant compounds were β-caryophyllene (31.33% ± 6.45%), δ-dodecalactone (26.88% ± 3.91%), and α-copaene (14.53% ± 2.83%). In the full flowering stage (Ⅳ), two monoterpenes, six sesquiterpenes, and one aliphatic compound were identified, including β-caryophyllene (69.02% ± 5.53%), α-copaene (10.28% ± 2.84%), and caryophylladienol-Ⅱ (7.39% ± 0.45%). In the loss of pedicel color stage (Ⅴ), six sesquiterpenes and one aliphatic compound were identified including α-copaene (31.99% ± 7.50%), nonanal (29.78% ± 9.10%), and formic acid, 3,7,11- trimethyl-1,6,10-dodecatrien-3-yl ester (17.86% ± 5.94%). In the wilting flower stage (Ⅵ), five sesquiterpenes and one aliphatic compound were identified, and the most abundant compounds were β-caryophyllene (56.74% ± 26.86%), α- copaene (20.02% ± 22.90%), and caryophylladienolⅡ (9.35% ± 3.65%).
Table 3. Percentage of volatile compounds identified in M. tenuifolia flowering stages using HS-SPME-GC-MS
yRelative contents (%) = (area under peak/total peak area) × 100.
xAll data are presented as the means ± SD (n = 3).
Bud (I); initial flowering stage (II); full flowering stage (III); loss of pedicel color stage (IV); wilting flower (V).
Expression Analysis of Floral Scent Genes
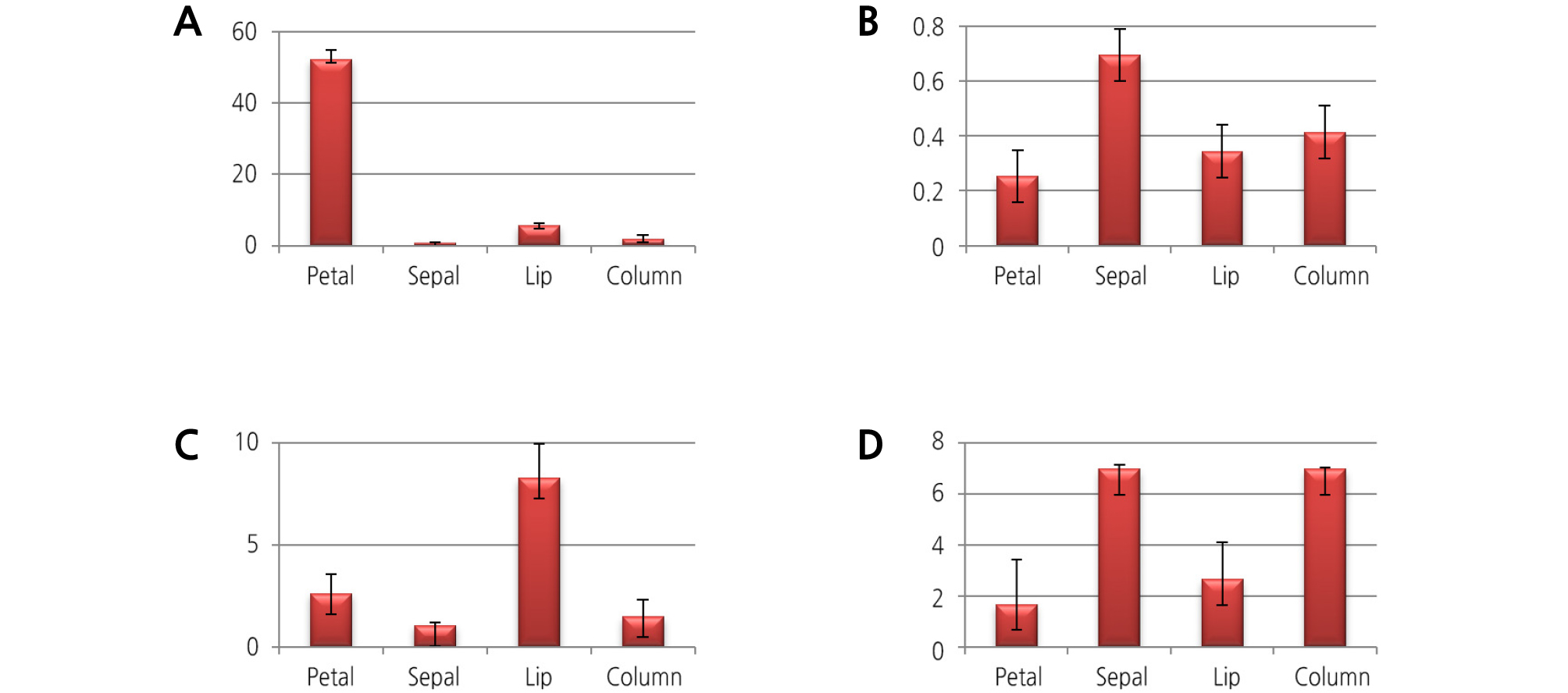
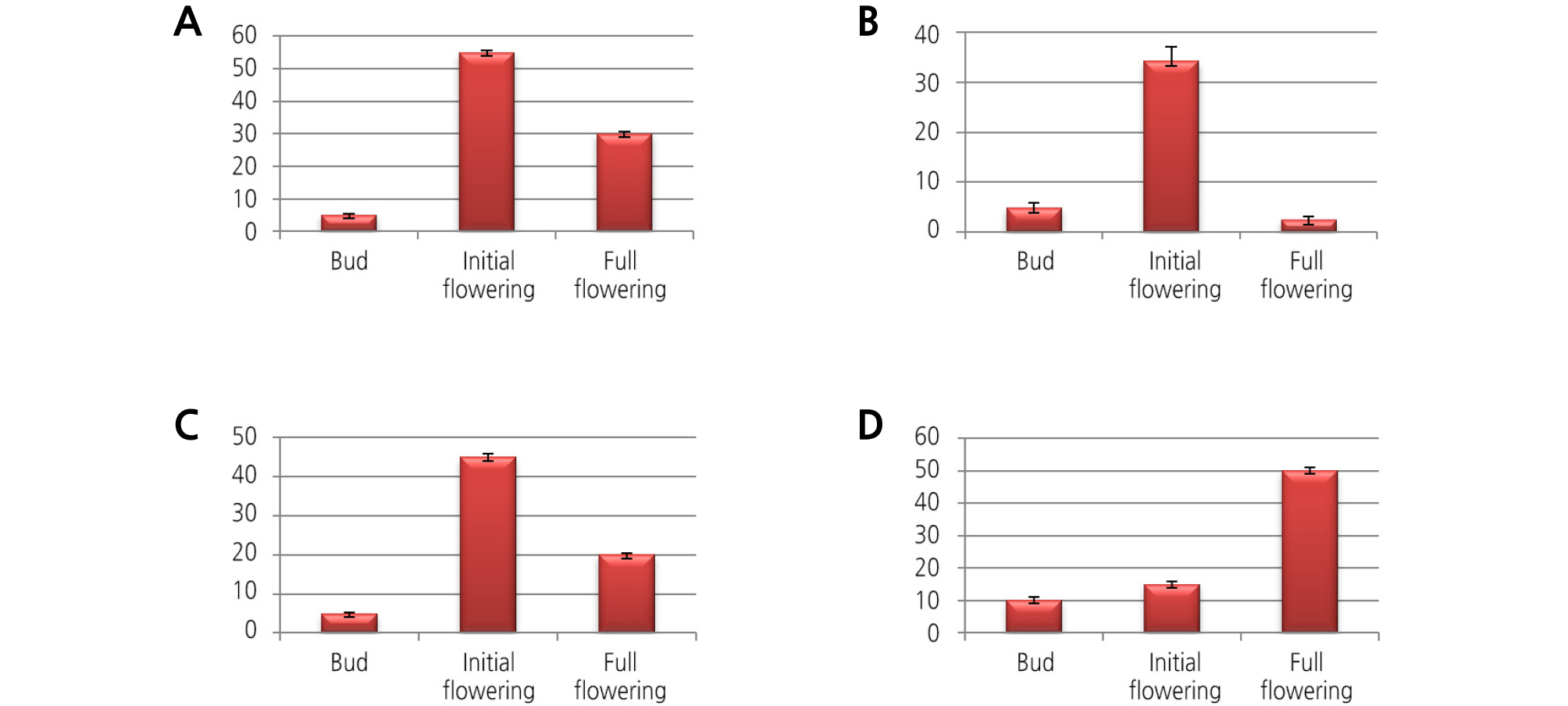
We analyzed the floral scent genes of different floral organs at various developmental stages of flowering by qRT-PCR. The results are shown in Figs. 1 and 2. The Y-axis indicates the expression fold change of the three biological replicates. Most of the floral scent genes for 1-deoxy-D-xylulose-5-phosphate synthase (DXS), farnesyl pyrophosphate synthase (FPS), geranyl diphosphate synthase (GDPS), and terpene synthase (TPS) were highly expressed at the initial flowering stage compared to the bud stage and the full flowering stage. Moreover, the sepal and petal flower organs showed the highest expression of floral scent transcript levels. Among the four genes, the FPS gene was highly expressed at the initial flowering stage with an approximately 35-fold increase in expression. The GDPS and DXS genes showed 14- and 3-fold increases at the initial flowering stage. The TPS gene was only expressed in the full flowering stage, with expression not detected at the other flowering stages. For the various floral organs, the expression of the FPS and TPS genes was highly upregulated in the sepal, followed by the petal, column, and labellum. The DXS and GDPS genes had the highest expression levels in the petals compared to the other floral organs. According these results, we think that the initial flowering stage of the flower, with the addition of the sepal and petal floral organs, is the main source for the floral scent for this plant.
Discussion
The main volatile compounds of M. tenuifolia, α-copaene and β-caryophyllene, were identified in every floral organ. The petal released the most volatile compound amount, as shown in Table 2. According to Tholl et al. (2005), the flower, petal, and sepal account for the floral volatile emission in Arabidopsis thaliana.
α-Copaene and β-caryophyllene, which are the major volatile compounds in M. tenuifolia, were identified at most of the flowering stages except the bud stage (I). The volatile compounds declined in content following the full flowering stage, and different compositions and amounts of volatile compounds were detected at different flowering stages. The epidermal cells of floral organs release fragrance through blooming when the cell growth is at a maximum and then decrease by senescence (Guterman, et al., 2002; Kolosova et al., 2001). In rose (Lee et al., 2008), Polianthes tuberosa (Kanani et al., 2017) and Lilium (Rho and Pak, 2001; Byun et al., 2007), volatile compound composition and release rates were also different as the flowering progressed. Also, in Arabidopsis, the gene encoding the caryophyllene synthase promoter is strongly active at the flower bloom, as reported by Tholl et al. (2005). The majority of the volatile compounds in M. tenuifolia were α-copaene, β-caryophyllene, and sesquiterpene, including δ-decalacton, a coconut scent (Perraudin et al., 2006). α-Copaene, a tricyclic sesquiterpene, was the main compound of Cedrelopsis grevei leaves, Annona reticulate, and Xylopia laevigata (Turkez et al., 2014a), and it has an effect on antioxidation (Turkez et al., 2014b). β-Caryophyllene is mainly extracted from herbs such as Cannabis sativa, rosemary (Ormeno et al., 2008), and Syzygium aromaticum (Ghelardini et al., 2001) and has an anti-inflammatory effect (Gertsch et al., 2008). Additionally, biological activities are attributed to this compound, such as anticancer (Legault and Pichette, 2007), antinociception (Katsuyama et al., 2012), anti-inflammatory (Cho et al., 2007), neuroprotective (Guimaraes-Santos et al., 2012), and antidepressant activities (Bahi et al., 2014). Eucalyptol and α-pinene, monoterpenes, are known to emit a sweet and fresh scent (Baek et al., 2016).
Floral scents and responsible genes are restricted to specific floral parts and developmental stages of the flower. When the flowers are ready to be pollinated, they emit more volatile compounds. After pollination, flowers reduce the synthesis of volatile compounds to prevent further visitors to nonpollinated flowers (Negre et al., 2003; Muhlemann et al., 2006; Rodriguez-Saona et al., 2011). Generally, the expression level of scent genes increases through the floral bud to the flowering stages, and in the senescence stage, volatile emission levels decrease in most flowers (Yue et al., 2015; Zheng et al., 2015). In M. tenuifolia as well, the floral scent genes for GDPS, DXS, FPS, and TPS were expressed at increasing levels from the bud stage to the flowering stage (Figs. 1 and 2). In Phalaenopsis, a new breeding cultivar, the terpenoid genes for LIS and GDPS gradually increased from the bud to the full flowering stage (Been et al., 2014). The FPS gene expression and volatile sesquiterpenoid level analyses in C. praecox flowers revealed that FPS may play a regulatory role in the sesquiterpenoid pathway in this species (Xiang et al., 2010). Tissue-specific emission of floral scents is a key characteristic of the majority of plant species. Among the floral parts, petals are the richest in volatile compounds, and most of the flower parts, including sepals, stamens, and pistils, contain a large number of biosynthetic enzymes; the highest gene expression is found in the flower parts (Dudareva et al., 1996; Murfitt et al., 2000; Nagegowda et al., 2010; Rodriguez-Saona et al., 2011). The DXS and DXR genes isolated from Rosa rugosa flowers also show consistent expression during development, from the budding to the withering stages (Feng et al., 2014). In Syringa oblata (Zheng et al., 2015), the expression of the DXS and DXR genes is positively correlated with the emission of volatile terpenoids during the full blooming of the inflorescence stage. According to these results, most of the floral scent gene expression gradually increases through the developmental stages.
These results show the different floral volatile compounds and gene expression for different floral organs and flowering stages of M. tenuifolia as in previous studies. These observations confirmed that unique floral scents are made with a variety of volatile compounds. Further work is needed to dissect the composition of the scent for breeding orchids for aromaticity and for the development of functional bioactive resources of the extract of plant organs, such as the flower, root, and bulb, for baseline perfume or cosmetic manufacturing through electroencephalography and cortisol determination.