Introduction
Materials and Methods
Culture Condition and Treatments
Treatment 1. Light Intensity
Treatment 2. Temperature
Treatment 3. Short-Term Temperature and Light Stress
Analysis of Growth Characteristics
Analysis of Chlorophyll & Functional Components
Statistical Analysis
Results and Discussion
Light Intensity Effects
Temperature Effects
Treatment 3. Short Term Temperature and Light Stress
Introduction
Since 2017, the sale of fresh-cut salads has increased 60% because of increased concern for health and because of their convenience (KRCB, 2019). The consumption of baby leaf vegetables also has increased. Baby leaf vegetables are about 10 cm high and contain 4 times more functional compounds, such as phenolic compounds, flavonoids, and minerals, than normal leafy vegetables. However, baby leaf vegetable varieties lack diversity because they use common leafy vegetables, such as lettuce, tat soi, red beet, and amaranth.
Wild vegetables are edible grasses and leaves that grow in the ground and on mountains. There are 480 species of wild vegetables, and only 30 kinds of crops are used in South Korea (RDA, 2018). In the past 5 years, due to the increasing concern about health and well-being, wild vegetable production and output are steadily increasing (KOSIS, 2018). Peucedanum japonicum Thunb. a wild vegetable, is a umbelligerae spp. and perennial herb that grows on the stream near the seashore. It is good for prevention and treatment of vascular dementia (Kim et al., 2013), lowering of blood pressure (Moon et al., 1983), and improvement of whitening and wrinkle (Kim et al., 2013). Okabe et al. (2011) reported that P. japonicum Thunb. could aid in weight control. However, most P. japonicum Thunb. studies have been conducted for medical research. Studies on the cultivation of wild vegetables grown in open fields or plastic houses were insufficient due to the shading, planting density, and soil type used (Song et al., 2016; Suwa et al., 2018; Jin et al., 2019).
Environmental conditions, light, temperature, water conditions, and CO2 are very important for cultivating vegetables. Among them, light intensity and temperature have a significant effect on the plant growth of functional material as well as on the growth (Savvas and Passam, 2002). Inadequate environmental stress produces reactive oxygen species (ROS) in plants, destroying plant DNA, RNA, proteins, and chlorophyll. To prevent destruction, plants produce natural antioxidants such as phenolic compounds to remove ROS. Therefore, environmental factors may be adjusted to increase functional substances. Lactuca indica L., a cultivated baby leaf vegetable, had increased total phenolic contents under high light levels (Kim et al., 2019), and lettuce can change its growth and functional contents with supplemental light (Li and Kubota, 2009). Lee et al. (2015) reported that the growth and functional contents of beet and Ssamchu were changed when the growth temperatures were 20°C, 25°C, and 30°C.
On the other hand, research is being conducted to increase the amount of functional substances in plants through short-term stress. Lee et al. (2014) reported that lettuce had increased total phenolic compounds without diminished growth when subjected to UV-a treatment for a short period of time. When 3-week-old kale was grown for 2 days at 4°C, a secondary metabolite production pathway was identified and phenolic compounds accumulated (Lee and Oh, 2015).
This study was conducted to investigate the growth responses and functional substance contents in P. japonicum Thunb. in response to optimum light intensity and temperature. We tried to determine whether functional contents could be increased before harvest without affecting growth through light intensity and low-temperature stress for 3 days.
Materials and Methods
Culture Condition and Treatments
P. japonicum Thunb., bred by Gangwon Agricultural and Extension Services, was used as plant material in this experiment. Before sowing, seeds were soaked in water over 6 hours and drained and stored in a 4°C refrigerator for 7 days. Thereafter, the seeds were sown in a 105-hole tray (hole size: W2.8× L2.8 × H4.0 cm) filled with horticultural soil (Baroker, Seoul bio Ltd., Eumseong, Korea) and left to grow for 16 days. Environment conditions for seedlings were air temperature of 25 ± 1°C with relative humidity of 60 ± 10% in a growth room using air conditioning (S-W138BAW, LG Electronics, Seoul, Korea) and a humidifier (NH-6, Hwajeun ENG., Daegu, Korea). Light intensity was set to 100 µmol·m-2·s-1 photosynthetic photon flux density (PPFD) using bar type LEDs (ZVAS, Sunghyun Hightech Co. Ltd., Hwaseong, Korea) and set at 16 h light / 8 h dark periods. Seedlings (plant height 5.8 cm, leaf number 1.9, fresh weight 0.2 g) were transplanted into a 72-hole plastic tray (Hole size: W4.0 × L4.0 × H5.0 cm) and treated with various light intensity and temperature treatments.
Treatment 1. Light Intensity
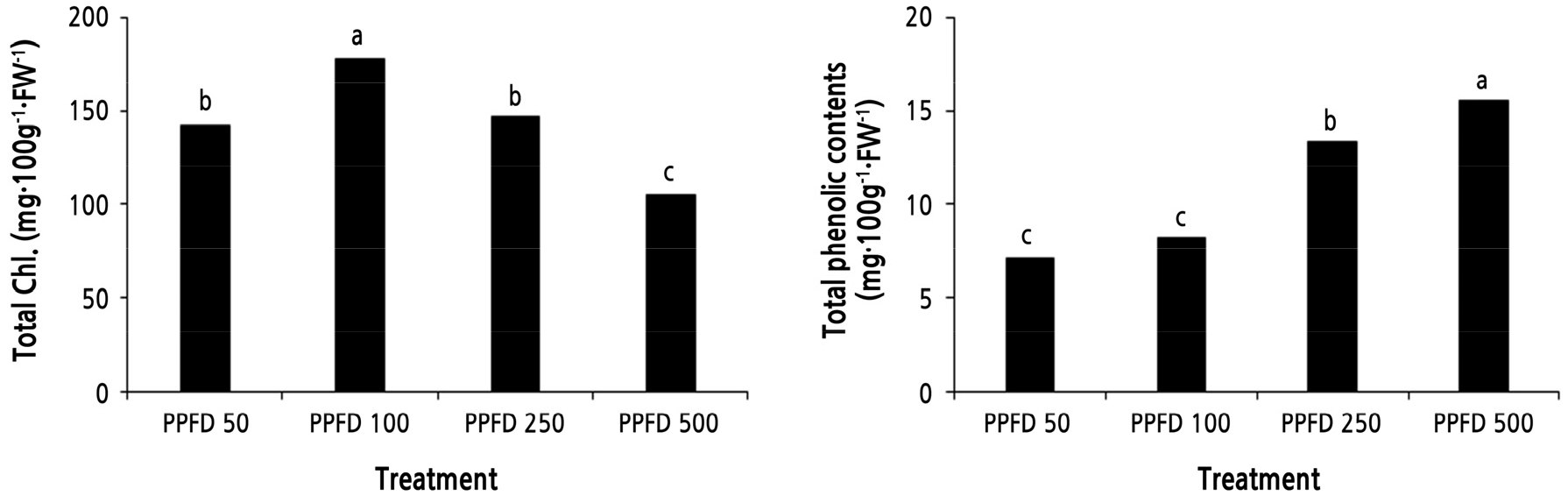
The plants were treated with four different light intensities, PPFD 50, PPFD 100, PPFD 250, or PPFD 500 µmol·m-2·s-1, using white LEDs with dimming control (NES-350-24, Mean Well Enterprises Co. Ltd., New Taipei, Taiwan) and a portable luxmeter (HD2102.2, Delta OHM, Padua, Italy). The light intensity was adjusted based on the plug tray. The cultivation temperature and relative humidity were maintained at 25 ± 1°C and 60 ± 10%, respectively, in a growth room. At 12 days after treatment, the baby leaves reached full size, and growth, chlorophyll contents, and total phenolic contents were examined.
Treatment 2. Temperature
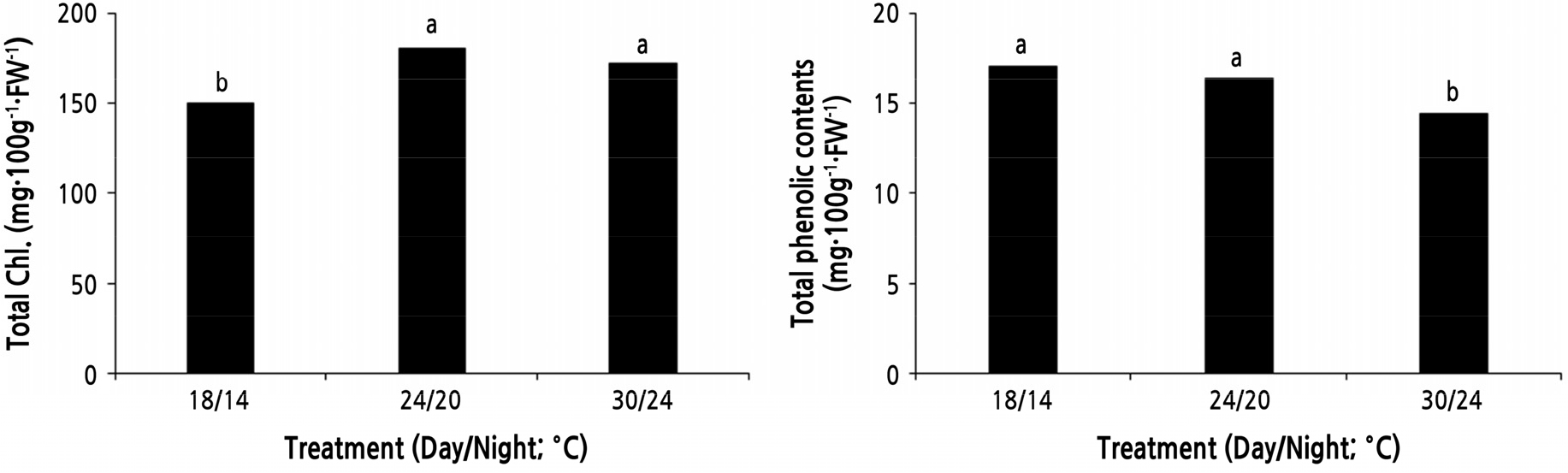
The plant was treated with three different growth temperatures (day/night), 18/14°C, 24/20°C, or 30/26°C using a closed-type plant production system (SJ-503PH, Sejong Scientific Co. Ltd., Bucheon, Korea). The cultivation relative humidity and light intensity were 60 ± 10% and 200 µmol·m-2·s-1, respectively. At 16 days after treatment, the baby leaves reached full size, and growth, chlorophyll contents, and total phenolic contents were examined.
Treatment 3. Short-Term Temperature and Light Stress
Environmental conditions for cultivation were an air temperature of 25 ± 1°C and relative humidity of 60 ± 10% with PPFD 150 µmol·m-2·s-1 in a growth room. After 15 days of cultivation, 3 days before harvest, the plants were subjected to five different treatments in a closed-type plant production system: 25/20°C - PPFD 200, 25/20°C -PPFD 500, 18/14°C -PPFD 200, 18/14°C -PPFD 500, or control (25°C -PPFD 150). At 3 days after treatment, growth, total phenolic content, and free radical activity (DPPH) were examined.
Analysis of Growth Characteristics
We measured leaf length and width, number of leaves, leaf area (Li-3100c, Li-cor Inc. Lincoln, NE, USA), shoot fresh weight, and shoot dry weigh. Specific leaf area (SLA) was calculated using the following equation (Eq. 1).
Analysis of Chlorophyll & Functional Components
Chlorophyll (Chl) contents were determined according to the method described by Mackinney (1941) with minor modifications. Briefly, torn fresh leaf (0.2 g) was used to extract Chl with 10 ml of 80% acetone at 4°C for 24 hours in dark conditions. Absorbance of the extract solution was measured at wavelengths of 645 and 663 nm with a UV spectrometer (UV-1800, Shimazu Corporation, Tokyo, Japan). Total Chl was calculated using Eq. 2. Total phenolic content (TPC) was determined according to the Folin & Dennis method described by Li and Kubota (2009) with minor modification. Fresh leaves (0.5 g) were used to extract TPC with 10 ml of 80% methanol at 4°C for 24 hours in dark conditions. Then 1 ml of the extract solution was mixed with 3 ml of distilled water, 1 ml of Folin & Ciocalteau’s phenol reagent, and 1 ml of water-saturated sodium carbonate solution. Fifty minutes later, absorbance of the mix solution was measured at a wavelength (WL) of 735 nm using a UV spectrometer. DPPH was determined according to the method described by Lee et al. (2003) with minor modifications. Fresh leaves (0.5 g) were used for extraction with 10 ml of methanol at 4°C for 24 hours in dark conditions. Then 0.9 ml of extract solution was mixed with 2.7 ml of 0.3 mM DPPH (2, 2-diphenyl- 1-picrylhydrazyl) solution. After incubation for 50 min in dark conditions at room temperature, absorbance of the solution was measured at a wavelength of 517 nm using a UV spectrometer. DPPH was calculated using Eq. 3.
Statistical Analysis
The experiment was replicated two times for all treatments and used a completely randomized design. Data were obtained from eight plants for plant growth characteristics and from four plants for Chl, TPC, and DPPH. The SAS package (statistical analysis system, version 9.4, SAS Institute Inc., Cary, NC, USA) was used for ANOVA (analysis of variance) and Duncan’s multiple range test (DMRT) at 5% for data analysis.
Results and Discussion
Light Intensity Effects
The optimum time for harvesting the P. japonicum Thunb. baby leaf vegetable was 12 days after treatments, and the plant height was 10 ‑ 12.7 cm (data not shown). In PPFD 250 and 500 treatments, leaf length and width were 3.4 cm and 5.3 ‑ 5.7 cm, respectively, larger than PPFD 50 and 100 treatments (Table 1). The number of leaves was 0.4 ‑ 0.9 less in the PPFD 50 treatment than the other three treatments. Leaf area increased with increasing light intensity and decreased after PPFD 250 treatment. Each plant species has a suitable light intensity. Plants grown in low light intensity have reduced growth due to low photosynthesis, whereas under excessive light levels, plants adapt by reducing leaf area, thickening leaves, and other physiological changes (Lichtenthaler et al., 2007). Song et al. (2016) reported that P. japonicum Thunb. had the best leaf growth at 35% shading treatment (PPFD 441.8 µmol·m-2·s-1) and poor growth at 75% shading treatment (139.5 µmol·m-2·s-1), which showed the same tendency as in our study. The shoot fresh weight was 1.0 g in the PPFD 250 and 500 treatments, 2.5 times heavier than the PPFD 50 treatment. The same trend was observed for shoot dry weight. Zha and Liu (2018) reported that chineses radish biomass is strongly dependent on light intensity, which is because plants produce and fix carbohydrates throughout photosynthesis, thus increasing their dry weight. The shoot dry weight of P. japonicum Thunb. increased when light intensity increased. SLA, meaning leaf thickness, was the smallest in the PPFD 50 treatment (581.1 cm2·g-1), and the value decreased with higher light intensity. Fan et al. (2013) reported that SLA increased when the light intensity decreased during the tomato seedling process. This was to increase the light absorption in the plant by maximizing the leaf area by thinning the leaf thickness.
Table 1.
Effects of light intensity on leaf length, leaf width, number of leaves, shoot fresh weight, shoot dry weight, and specific leaf area (SLA) of Peucedanum japonicum Thunb.
| PPFD (µmol·m-1·s-1) | Leaf | Shoot weight (g/plant) | SLA (cm2·g-1) | |||||
| Length (cm) | Width (cm) | Number | Area (cm2) | Shoot | Dry | |||
| 50 | 2.2 cz | 3.6 c | 3.0 b | 12.7 c | 0.4 c | 0.03 c | 581.1 a | |
| 100 | 2.8 b | 4.9 b | 3.4 ab | 24.5 b | 0.8 b | 0.08 b | 332.6 b | |
| 250 | 3.4 a | 5.7 a | 3.9 a | 30.7 a | 1.0 a | 0.12 a | 271.3 b | |
| 500 | 3.4 a | 5.3 ab | 3.4 ab | 28.7 ab | 1.0 a | 0.14 a | 208.4 b | |
Total Chl and TPC differed according to light intensity treatments (Fig. 1). Total Chl was highest in the PPFD 100 treatment and lowest in the PPFD 500 treatment. Chl tends to decrease when the plant is under stress (Iglesias et al., 2006). Because the PPFD 100 treatment created the least light stress, the total Chl seemed to be high, and the PPFD 50 treatment, which is a very low light level to accumulate energy through photosynthesis, had low total Chl content. On the other hand, the TPC increased with higher light intensity. The maximum TPC was 15.6 mg·100g-1·FW-1 in the PPFD 500 treatment, which was 2.2 times higher than the PPFD 50 treatment. When Indian lettuce ‘Sunhyang’ was grown for 12 days after light intensity treatments, the TPC was 2.1 times better than the PPFD 500 treatment (47.4 mg·100g-1·FW-1) compared to the PPFD 50 treatment (Kim et al., 2019). Pérez-López et al. (2018) reported that environmental stress, such as high light intensity, prevents the growth of lettuce but increases functional substances and enhances antioxidant capacity.
Temperature Effects
Sixteen days after temperature treatments, the leaf length, width, and number of the 24/20°C treatment were 4.4 cm, 7.1 cm, and 4.8 ea, respectively, larger than those of the other two treatments (Table 2). Differences in leaf length, width, and number also showed differences in leaf area. Shoot fresh weight and dry weight were higher in the 24/20°C treatment with better leaf growth. Temperature affects plant growth by regulating the rate of enzymatic reactions in the plant, and growth rate increases to an appropriate temperature and then slows down at the temperature. In our study, P. japonicum Thunb. hadincreased leaf growth, fresh weight, and dry weight in the 24/20°C treatment and then decreased in high-temperature treatments. The SLA was the highest at 224.7 cm2·g-1 in the 24/20°C treatment. Therefore, the proper cultivation temperature of P. japonicum Thunb. is 24/20°C, and PPFD 200 µmol·m-2·s-1 is the suitable light intensity for a faster leaf growth rate and thinner leaves than the other two treatments.
Table 2.
Effects of growth temperature on leaf length, leaf width, number of leaves, shoot fresh weight, shoot dry weight and specific leaf area (SLA) of Peucedanum japonicum Thunb.
| Temperature (Day/Night°C) | Leaf | Shoot weight (g/plant) | SLA (cm2·g-1) | |||||
| Length (cm) | Width (cm) | Number | Area (cm2) | Shoot | Dry | |||
| 18/14 | 3.6 bz | 5.8 a | 4.2 b | 39.2 b | 1.6 ab | 0.19 b | 207.3 b | |
| 24/20 | 4.4 a | 7.1 a | 4.8 a | 61.2 a | 2.1 a | 0.27 a | 224.7 a | |
| 30/26 | 3.1 b | 5.5 a | 4.6 b | 40.4 b | 1.4 b | 0.20 b | 206.5 b | |
As a result of measuring the total Chl and TPC according to temperature (Fig. 2), total Chl at 24/20°C and 30/26°C was 255.9 ‑ 268.1 mg·100g-1·FW-1, which was higher than the 18/16°C treatment. In the case of Pica asperata and Pinus tabulaeformis, the total Chl increased at high temperature (Zhao and Liu, 2009), and Vigna radiata and Vigna unguiculata showed different results (Hamid et al., 2009) indicating that plants have different physiological responses (Kim and Han, 2015). In this study, Chl was decreased at low temperature. TPC was higher in the 18/14°C and 24/20°C treatments (150 ‑ 181 mg·100 g-1·FW-1) than in the 30/26°C treatments (143 mg·100 g-1·FW-1). In plants, the accumulation of antioxidants increases due to environmental stress (Dixon and Paiva, 1995). Lettuce treated at 40°C and 4°C has increased quercetin and luteolin contents, thus increasing TPC (Oh et al., 2009). Our results showed that TPC accumulation was higher in low temperature than high temperature conditions.
Treatment 3. Short Term Temperature and Light Stress
When treated with temperature and light intensity 3 days before harvest, the leaf length and width of P. japonicum Thunb. were 2.4 ‑ 2.6 cm and 4.3 ‑ 4.6 cm, respectively, and the number of leaves and fresh weight were 2.8 ‑ 3.0 and 0.5 ‑ 0.6 g, respectively (data not shown). There was no difference among treatments.
However, TPC and DPPH showed differences among the treatments despite short-term treatments (Fig. 3). TPC in the 18/14°C treatments was 17.1 ‑ 20.4 mg·100 g-1·FW-1, 1.6 ‑ 2.1 times higher than the control and 25/20°C treatments. It was 5 mg·100g-1·FW-1 higher than in the PPFD 500 treatment and not significantly different form 18/14°C treatments. TPC was highly significant in the short-term low-temperature treatment (0.001 < p < 0.01), while the short-term high light intensity treatment was not significant. When kale ‘Manchoo’ was treated at 4°C for 2 days, caffeic acid, ferulic acid, and kaempferol content were higher than control cultivation (25°C), and it was confirmed that the signal fraction acts to generate secondary metabolites such as singlet oxygen and hydrogen peroxide in plants (Lee and Oh, 2015). In this study, it was also confirmed that 3-day 18/14°C treatments can enhance TPC. DPPH showed a similar tendency to TPC and low temperature and also showed significant correlation with low temperature, high PPFD, and low temperature × high PPFD. Palaniswamy et al. (1997) reported that when watercress was treated with high light intensity (PPFD 435 µmol·m-2·s-1) for 1 week before harvest, the content of phenethyl isothiocyanate, a phytochemical that inhibits several types of cancer, increased. In addition, Jin et al. (2015) reported that light treatment after broccoli harvest improves the TPC and also increases antioxidant scavenging ability.
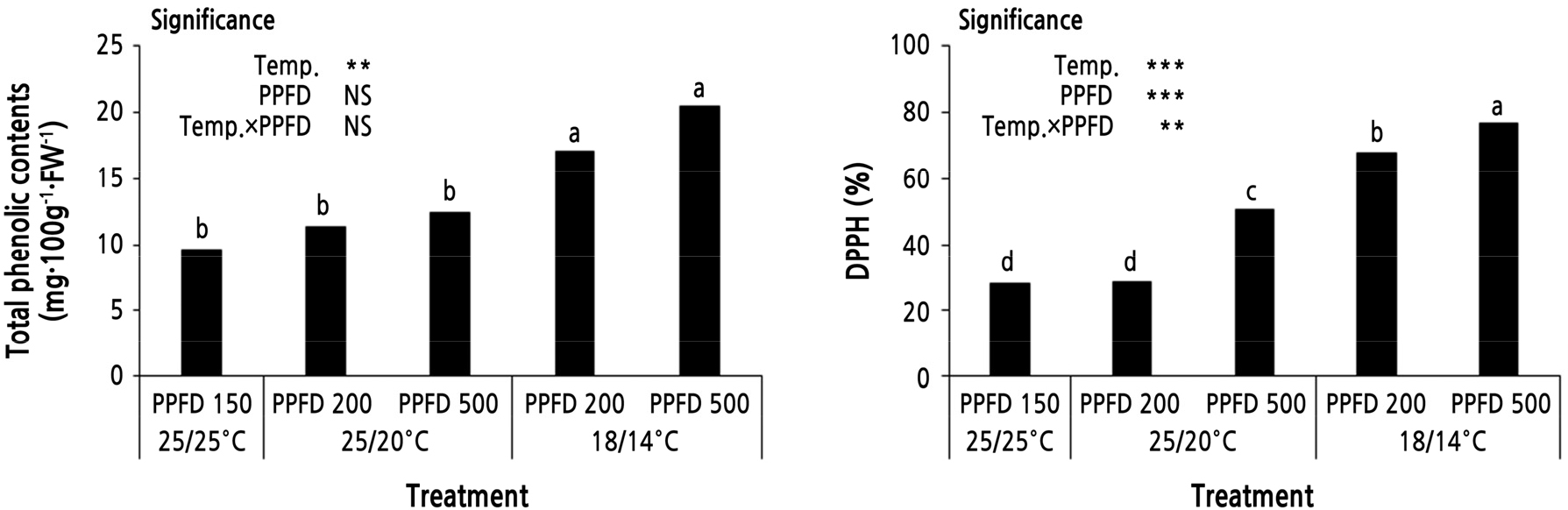
Fig. 3
Effects of low temperature and short-term high light intensity on total phenolic content and free radical activity (DPPH) of Peucedanum japonicum Thunb. The different letters in each column indicate significant difference by DMRT at p < 0.05(n = 4). NS, **, *** mean non-significant, significant at p < 0.01 and 0.001, respectively.
In summary, light intensity and temperature treatment affected the growth and functional substances of the P. japonicum Thunb. baby leaf vegetable. For growth and functional substances, the optimum light intensity of P. japonicum Thunb. was 12 days in PPFD 250 and temperature of24/20°C. Cultivation at above PPFD 250 or at low temperature increases the TPC but may lead to poor growth rate. However, the TPC compounds and DPPH can be increased at a low temperature of 18/14°C than high light treatment just before harvesting. It has been concluded that short-term stress at low temperature, rather than high light intensity, will yield high-quality P. japonicum Thunb. with high phenolic compounds.