Introduction
Materials and Methods
Experimental Site, Soil Properties, and Climate Data
Crop Management, Experimental Layout, and Irrigation Treatments
Yield, Fruit Quality, and Shoot Dry Weight
Physiological and Biochemical Parameters
Statistical Analysis
Results
Yield, Fruit Quality, and Shoot Dry Weight
Chlorophyll Index and Phenolic Compounds
RWC, Electrolyte Leakage, and Proline Content
Discussion
Introduction
Watermelon [Citrullus lanatus (Thunb.) Matsum. et Nakai] is one of the most economically important fruits that is cultivated in many parts of the world. Iran is the third largest watermelon producing country after China and Turkey, producing 3.8 million tons from 132,464 ha each year (Fao, 2016). However, most of this production owes to improved cultivars of foreign origin. There is a high level of genetic diversity among Iranian watermelon landraces, such that those grown as rainfed and seedy watermelon in different regions of Iran under drought stress still result in high yields.
Many countries are facing drought stress due to global warming and climate change, especially in the west of Asia. Water deficit conditions have reduced the average yield of crops by more than 50% (Wang et al., 2003) by altering cellular water potential and stomatal conductance, inhibiting photosynthesis, and enhancing the accumulation of reactive oxygen species (ROS) (Kumar et al., 2017). Breeding and genetic transformation methods could be used to create and improve crop varieties resistant to water deficient conditions. While efforts have been limited due to the genetically and physiological complexity of the trait (Chaves et al., 2003), improved cultivars could alternatively be grafted onto resistant rootstocks to overcome drought conditions.
Vegetable grafting has been used in horticulture practices for more than 50 years in different regions of the world. The technique was first used to prevent damage caused by soil borne pathogens (Oda, 2002). At present, this technique is used to improve the tolerance of vegetables to alkaline condition (Colla et al., 2010), potassium deficiency (Huang et al., 2013), boron and copper toxicity (Edelstein et al., 2005), salinity stress (Colla et al., 2012; Colla et al., 2013; Yang et al., 2015), and drought stress (Rouphael et al., 2008; Etemadipoor, 2015; Özmen et al., 2015; Penella et al., 2017).
A number of commercial rootstocks exist including bottle gourd and an interspecies hybrid between C. maxima and C. moschata that have been promoted for watermelon grafting (Davis et al., 2008). Both of them are not perfect and show some undesirable effects on fruit size, shape, flesh quality, peel thickness, and sugar content (Edelstein et al., 2014). Bigdelo (2016) and Parkhideh (2017) have tried to improve drought tolerance of watermelon by grafting onto bitter apple (Citrullus colocynthis). Additionally, Rouphael et al. (2008), Özmen et al. (2015), and Etemadipoor (2015) evaluated the effects of Cucurbita rootstocks on watermelon yield and quality under drought stress. To date, Iranian rainfed and seedy watermelon landraces have not been investigated as rootstocks for watermelon faced with drought stress. Considering that the region has faced consecutive droughts and high levels of water consumption during watermelon production processes, we decided compare rainfed and seedy watermelons with control and Shintoza (commercially well known) as rootstocks to overcome water shortage problems. We hypothesized that these local landraces when cultivated in arid areas under deficit irrigation regimes, could increase drought tolerance potential without the negative effects of Cucurbita rootstocks on fruit quality.
Materials and Methods
Experimental Site, Soil Properties, and Climate Data
Experiments were carried out during two consecutive growing seasons in 2016 and 2017 in an experimental field at the University of Tabriz located in Tabriz, in the northwest part of Iran (latitude: 38° 1' 22.23" N, longitude: 46° 25' 9.38" E). Physical and chemical properties of the soil in the experimental field are presented in Table 1. The percentage of sand, silt, and clay were measured using a hydrometer (Gee and Bauder, 1979); wet oxidation method was used to measure soil organic matter content (Nelson and Sommers, 1996); and bulk density was measured by core method (Blake and Hartage, 1986). Soil pH and electrical conductivity (EC) in saturated paste extracts were determined using a pH meter (Hach Ec30; Loveland, USA) and and EC meter (Test 0240; Keison Products, Chelmsford, UK), respectively (Richards, 1969). Soil moisture content at field capacity (FC) was determined at -10 KPa by using a hanging-water-column method (Dane and Hopmans, 2002). Monthly climate data during the two consecutive growing seasons are shown in Table 2.
Table 1. The physical and chemical properties of experimental soil
| Sand (%) | Silt (%) | Clay (%) | Organic matter (%) | pH | Bulk density (g‧cm-3) | EC (dS‧m-1) | FC (%) |
| 62.8 | 22.5 | 14.7 | 1.2 | 8.7 | 1.3 | 1.5 | 18.0 |
Table 2. Climatic parameters of two growing seasons (2016 and 2017)
Crop Management, Experimental Layout, and Irrigation Treatments
Watermelon (Citrullus lanatus (Thunb.) Matsum. et Nakai cv. Crimson sweet F1), selected as the most popular cultivar in Iran, was used as a scion, and as a control (un-grafted treatment). Seeds of local rainfed and seedy watermelon were collected from different areas of Iran (Table 3) as the rootstocks, as well as ‘Shintoza’ (C. maxima × C. moschata) seedlings, a commercial and widely used hybrid rootstock of watermelon. Seeds of local landraces were sown 16 days before grafting, while those of ‘Shintoza’ were sown 9 days before grafting, and those of scion 10 days before grafting. Forty-eight cell plastic trays filled with peat and perlite (1:3) were used for planting. Seedlings were grafted by splice grafting method (Lee, 1994).
Table 3. Local rootstocks of watermelon and their origin
Grafted and un-grafted plants were transplanted to the field with sandy loam soil on 26 June 2016 (season 1) and 28 June 2017 (season 2). A starter fertilizer (20N-20P-20K, 5 g per plant) was applied through the irrigation water three times after transplanting. Additional fertilizer containing micronutrients and 15N-5P-30K (3 g per plant) was applied every two weeks during the growing seasons. In both growing seasons, powdery mildew was controlled by foliar treatment of Orthocide 50% fungicide at the labeled rate. weed control were done with hand hoeing during the growing seasons.
Treatments were conducted by a split plot combination of three irrigation regimes based on field capacity (FC) [0.8FC-FC (T1), 0.6FC-0.8FC (T2), and 0.3FC-0.6FC (T3) (in each treatment the first letter represents the lower limit of the specified range and the second represents the upper limit of the desired range of soil moisture)] as the main plots and five graft combinations were arranged into subplots. Each experimental unit consisted of two rows in two sides of a furrow, 4 m in length, with 50 cm in-row spacing and 70 cm row spacing containing 15 plants. The treatments were defined in a randomized complete block design with three replicates and three samples.
Transplanted watermelons were regularly irrigated by furrow method manually (every two days) for two weeks to enhance root system establishment. A time domain reflectometer (TDR) device (Soil Moisture Equipment Corporation, Santa Barbara, USA) was used to measure and maintain soil moisture content at the desired ranges. The 45 cm TDR wave guides were vertically inserted into three points in each plot and their position was permanently fixed in the soil during the entire experimental period. Soil moisture content was measured once every two days. When the moisture content in the plots decreased to the lower limit of the specified range, water was added to the plots to raise the moisture to the upper limit of the desired range. The volume of water required was calculated with equation 1.
| $$\mathrm V=\mathrm{aD}(\theta_{\mathrm V2}\;-\;\theta_{\mathrm V1})$$ | (1) |
Where: V = volume of water required (cm3), aD = depth (45 cm) and cross-section area (30,000 cm2) of the plots, θ V2 = upper limit of the selected moisture range (cm3·cm-3), θ V1 = moisture content (cm3·cm-3) at the time measured using a TDR (Zarehaghi et al, 2015).
Yield, Fruit Quality, and Shoot Dry Weight
In both seasons, fully mature fruits were harvested on 8 September 2016 (70 days after transplanting), and on 9 September 2017 (71 days after transplanting). The yield was expressed as kg per plant. In both seasons, 6 representative fruits per treatment were evaluated for the following fruit quality parameters: total soluble solids (TSS) (°Brix), % titratable acidity (TA), rind thickness (mm), and flesh firmness (N). Two days after harvest the filtered liquid of the fruit mesocarp extract was used to measure TSS with a refractometer (Atago, Japan), and reported in °Brix at 20°C. TA was measured by potentiometric titration with 0.1 M NaOH up to pH 8.1 using 15 mL of juice. Results were expressed as the percentage of malic acid in the juice. Fruit firmness was evaluated with a penetrometer (8-mm diameter plunger) (Force Gauge, USA). For determination of shoot dry weight, total epigeous organs were dried in oven set to 70°C for 72 hours, after which they were weighed.
Physiological and Biochemical Parameters
Chlorophyll index was measured by SPAD set (SPAD 502, Minolta, Japan) during the development of plant leaves. Phenolic compounds were determined by Folin–Ciocalteu (F&C) reagent using the method of Singleton and Rossi (1965). The level of electrolyte leakage was calculated based according to Lutts et al. (1995) and relative water content (RWC) according to Schonfeld et al. (1988). Proline concentration was determined according to the method described by Bates et al. (1973).
Statistical Analysis
Statistical analyses were performed using statistical analysis system (SAS) version 9.1, and the mean values were evaluated using Duncan’s multiple range test (p < 0.01).
Results
Yield, Fruit Quality, and Shoot Dry Weight
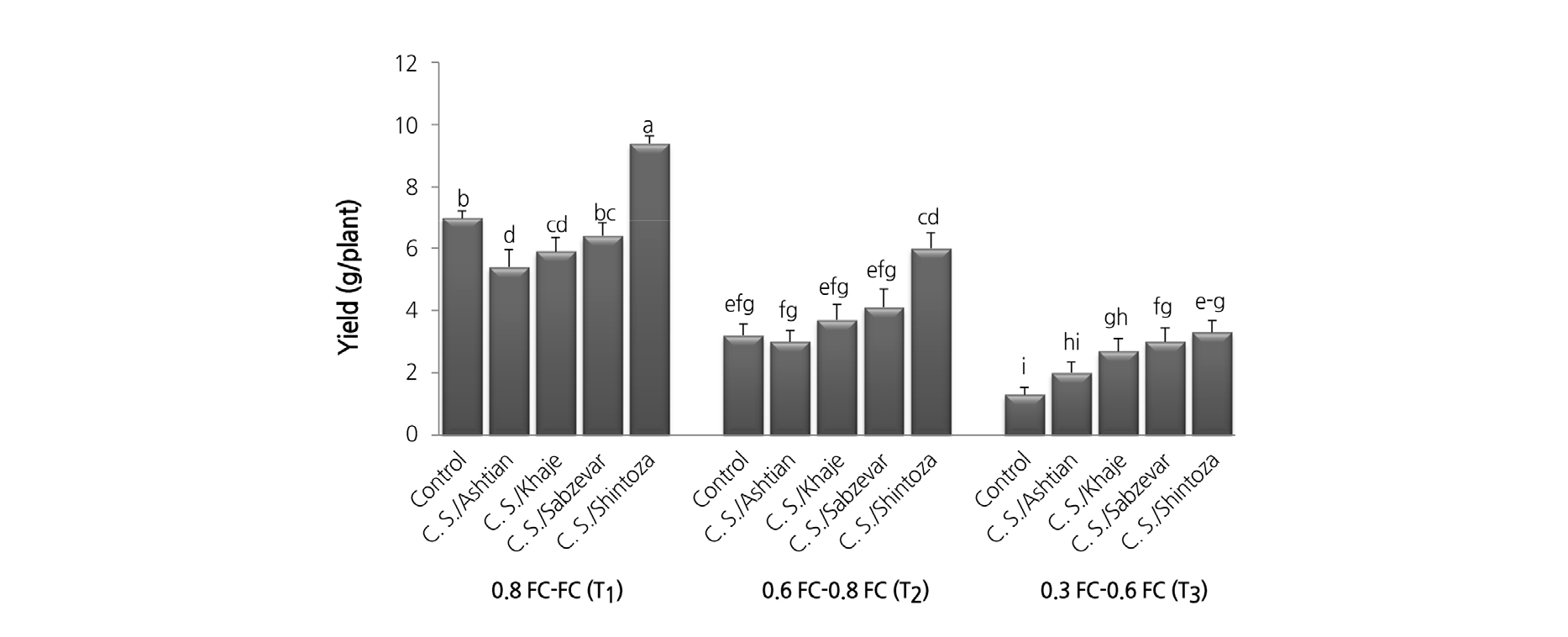
The results showed that yield (p ≤ 0.05), and shoot dry weight (p ≤ 0.01) were significantly influenced by interaction effects between irrigation regime and graft combination (GC) in both growing seasons. Overall, the yield production linearly decreased with drought stress (Fig. 1). The highest yield was observed for ‘Shintoza’ GC at T1 (9.4 kg/plant), and the lowest was in control GC (un-grafted plants) at T3 (1.4 kg/plant).
According to the results, no significant differences were found between graft combinations except for ‘Shintoza’ GC at T1 and T2 separately. Shintoza, Sabzevar, and Khaje GC resulted in significantly higher yields in comparison to the control at T3, while un-grafted plants as well as Ashtian displayed similar levels. There were significant differences between various graft combinations for TSS, TA, peel thickness, and flesh firmness (Table 4). Furthermore, peel thickness was affected by irrigation rates. Comparison of means showed that Shintoza GC exhibited significantly different results for the aforementioned traits compared to other graft combinations.
Table 4. Main effect of irrigation regimes based on TDR, and graft combination on total soluble solids (˚Brix), titratable acidity (%), peel thickness (mm), and flesh firmness (N) of watermelon fruits
ns, *, ** Non significant or significance at p ≤ 0.05 and p ≤ 0.01, respectively.
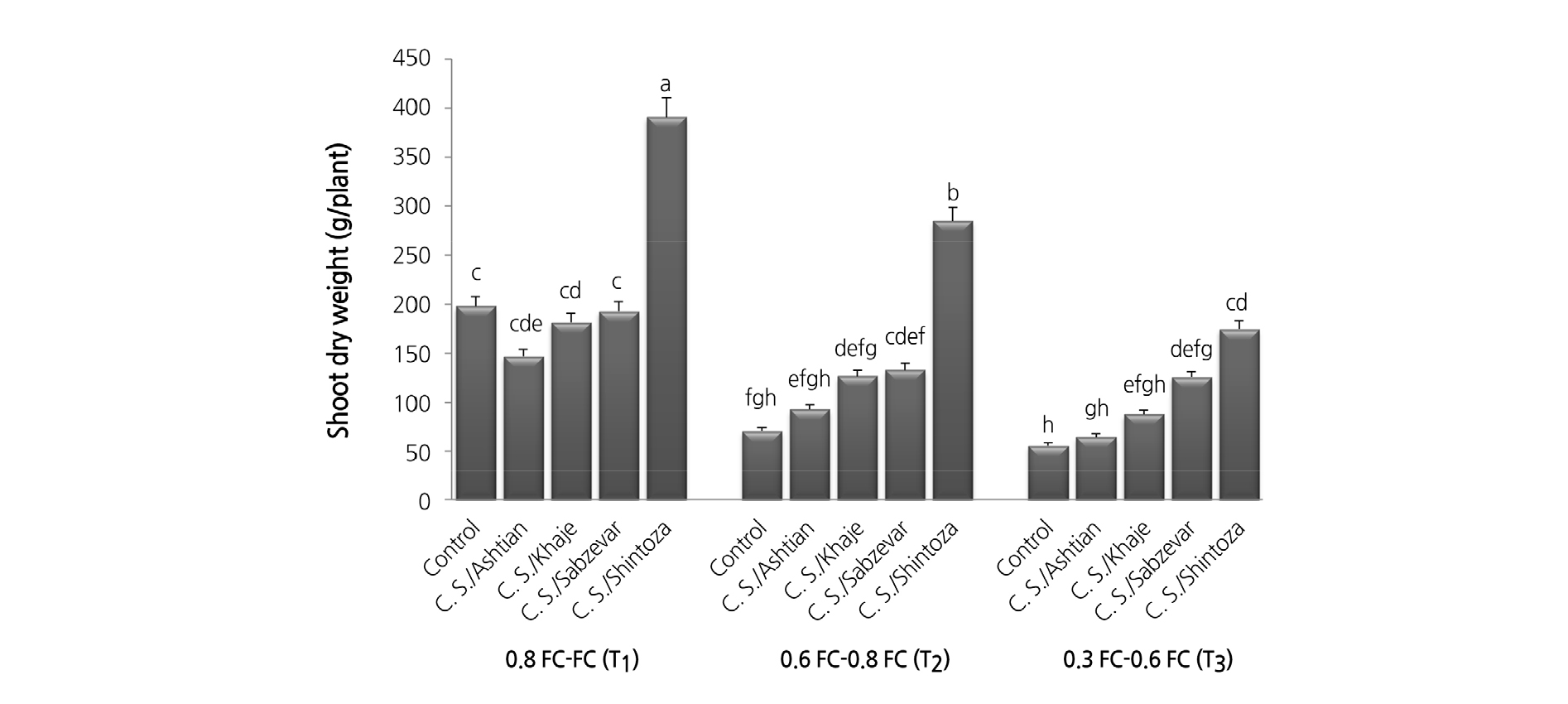
Shoot dry weight was significantly reduced under drought stress (Fig. 2). Compared with the control at T3 (55.6 g/plant), the highest shoot dry weight (390 g/plant) was obtained in Shintoza GC at T1. No significant difference was observed in shoot dry weight between grafted plants onto Sabzevar and Khaje rootstocks at T1 and T2. However, control, Ashtian, and Khaje GC at T3 along with control and Ashtian GC at T2 were placed in the same group. Plants grafted onto Shintoza and Sabzevar rootstocks significantly increased the shoot dry weight compared with the un-grafted plants at T3.
Chlorophyll Index and Phenolic Compounds
According to the data, chlorophyll index and phenolic compounds were significantly affected by the effects of irrigation regime and grafting combination (p ≤ 0.01), but were not significantly affected by year (Y), Y × I, Y × G, I × G, and Y × I × G (Table 5). Drought stress in T3 decreased the chlorophyll index of watermelon (53.9) compared with T1 and T2 (60.4 and 60.6). Furthermore, a high chlorophyll index was obtained in Shintoza GC (62) and Sabzevar GC (60), compared with control (54.5) and Ashtian GC (55.6). Phenolic compounds were significantly affected by different irrigation regimes. The highest (6.3 mg GA/g FW) and lowest (4.7 mg GA/g FW) phenolic compound contents were recorded in T1 and T3, respectively. The levels of phenolic compounds were significantly affected by grafting combinations. Plants grafted onto Shintoza and Sabzevar rootstocks generated maximum amounts of 6 and 5.8 mg GA/g FW, respectively, compared with control (5.1), Ashtian (5.3), and Khaje GC (5.4).
Table 5. Main effect of irrigation regimes based on TDR, and graft combination on chlorophyll index, RWC, electrolyte leakage, proline, and phenolic compounds of watermelon plants
ns, *, ** Non significant or significance at p ≤ 0.05 and p ≤ 0.01, respectively.
RWC, Electrolyte Leakage, and Proline Content
Comparison of means showed that RWC was significantly affected by irrigation regime and grafting combination (p ≤ 0.01) (Table 5). Also, electrolyte leakage and proline content were significantly affected by irrigation regime (p ≤ 0.01), while Y, G, Y × I, Y × G, I × G, and Y × I × G did not show significant effects on electrolyte leakage and proline content. The RWC values in watermelon leaves at T3 (76.8%) and T2 (81.1%) were significantly reduced by drought stress, compared with T1 (86.2%). Un-grafted plants had the lowest values of RWC compared with watermelons grafted onto Shintoza, Khaje, and Sabzevar rootstocks. Electrolyte leakage was only affected by T3 (48.3%) compared to T1 (42.7%) and T2 (43.6%). However non-significant differences in proline accumulation were found between two levels of drought stress and grafting combinations, but proline concentrations increased in response to drought by 9.44 and 9.02 mM·g-1 FW at T2 and T3, compared to 6.79 mM·g-1 FW at T1.
Discussion
Drought stress causes significant reductions in growth and yield of crops (Chaves et al., 2003; Proietti et al., 2008; Rouphael et al., 2008). In the present study, the yield in watermelons grafted onto Cucurbita rootstock Shintoza was significantly higher than un-grafted plants in different irrigation treatments. This finding was consistent with the research results conducted by Yetisir and Sari (2003), Proietti et al. (2008), Rouphael et al. (2008), Etemadipoor (2015), and Özmen et al. (2015). Edelstein et al. (2014) evaluated five wild watermelon accessions as potential rootstocks, with two Cucurbita rootstocks, and reported that watermelon grafted onto Cucurbita rootstock ‘TZ-148’ had more yield compared to un-grafted plants. However, they reported that there were no significant differences between watermelons grafted onto five wild watermelon accessions and un-grafted plants, whereas we found that the yield significantly increased in plants grafted onto Sabzevar rootstock compared with control GC at T3 (Fig. 1). Increased vigor of Shintoza GC at T1, T2, and T3, as well as Sabzevar GC at T3, indicated by their higher yields and shoot dry weights (Figs. 1 and 2), are suggestive of positive interactions between scion and rootstock, increased absorption of nutrients and water through the elaborate root system of the rootstock (Lee, 1994; Ruiz et al., 1997; Pulgar et al., 2000), and potential increases in the production of endogenous hormones by roots (Zijlstra et al., 1994).
Negative effects of Cucurbita rootstocks on fruit quality have also been shown previously (Lee, 1994; Lee and Oda, 2003; Edelstein et al. 2014). According to Table 4, fruit quality aspects including TSS, TA, peel thickness, and flesh firmness were different only in Shintoza GC compared to the other treatments. In other words, Iranian rainfed and seedy watermelon rootstocks had no obvious negative effect on fruit quality.
Maintenance of chlorophyll content has been regarded as a desirable trait under drought stress, as it reflects reduced pigment photooxidation and chlorophyll degradation (Anjum et al., 2011). Significant increases in chlorophyll index were observed for plants grafted onto Shintoza, Sabzevar, and Khaje rootstocks (Table 5), which may reflect increased root vigor and absorption of water and other nutrients essential for the biosynthesis of chlorophyll (e.g., N and Mg).
Phenolic compounds have antioxidative properties through their ability to release hydroxyl (–OH) group hydrogen atoms (Weidner et al., 2009). However, Alan et al. (2017) reported that phenolic compounds in watermelons were not affected by graft combination. In contrast, Evrenosoğlu et al. (2010) found that phenolic compounds in watermelon grafted onto Cucurbita rootstocks were higher than in un-grafted plants. According to our results (Table 5), Shintoza GC produced the highest level of phenolic compounds followed by Sabzevar GC.
Leaf RWC plays a key role in drought stress tolerance by inducing osmotic adjustments via the accumulation of osmoprotectants (Barnabás et al., 2008; Zhang et al., 2012). In this study, RWC in the leaves significantly decreased in response to drought stress (Table 5). Similar results were reported by Rouphael et al. (2008) in watermelon and by Barzegar et al. (2017) in melon, although Roupheal et al. (2008) reported that no differences were found in RWC between grafted and un-grafted watermelon under various irrigation treatments. The results of this study showed that Shintoza, Sabzevar, and Khaje GC significantly increased RWC compared to the control (Table 4). Reduced RWC in plants under drought stress may be related to plant vigor reduction (Liu et al., 2002).
Maintaining the integrity of membranes under drought stress is another major component of drought tolerance in plants (Bajji et al., 2002). Based on the results of this study, severe drought stress (T3) impaired the cell membranes of watermelons leaves and significantly enhanced the level of electrolyte leakage (Table 5).
It also appears that proline accumulation plays an important role in drought stress tolerance through ROS scavenging, protection of important cellular macromolecules, and maintenance of the cell water balance (Verbruggen and Hermans, 2008; Farooq et al., 2009). Kawasaki et al. (2000) reported that wild watermelon accumulated citrulline followed by glutamate and arginine, instead of proline and glycinebetaine, while Dasgan et al. (2009) reported that citrulline plays a more effective role compared to proline in response to salinity stress in two Turkish melon landraces. We found that proline accumulation increased significantly in response to drought stress compared to T1, while no significant differences were found between two levels of drought stress or among grafting combinations. This suggested that the watermelons used in this study may generate other compatible solutes, such as citrulline instead of proline.
We found that plants grafted onto Shintoza rootstocks increased yield and shoot dry weight at T1, while higher yield and dry biomass were obtained in Sabzevar GC when compared with the control at T3. On the other hand, fruit quality features of Shintoza GC were negatively influenced compared to the other graft combinations. Furthermore, no significant effects were observed in fruit quality parameters of those grafted onto rainfed and seedy watermelons, which were similar to the controls. The high levels of yield, shoot dry weight, chlorophyll index, phenolic compounds, and RWC of plants grafted onto Shintoza and Sabzevar rootstocks suggest to us that an Iranian rainfed and seedy watermelons Sabzevar would be regarded as a potential rootstock for watermelon under drought stress without any detrimental effects on the fruit quality.