Introduction
Materials and Methods
Plant Materials and Cultivation
Analysis of Photosynthetic Rates and Chlorophyll Fluorescence
Analysis of Root Activity
Experimental Design and Statistical Analysis
Results
RH in Korea During the Cold Growing Season
Photosynthesis Parameters of Leaves of Two Strawberry Cultivars Grown Under Different RH Conditions
Chlorophyll Fluorescence OJIP Transient Parameters of Leaves of Two Strawberry Cultivars Grown Under Different Relative Humidity Conditions
Root Activity of Two Strawberry Cultivars Grown Under Different Relative Humidity Conditions
Discussion
Introduction
Protected horticulture cultivation in Korea is mainly intended to enable greenhouse crops to thrive during cold weather, primarily from early winter to early spring, by carefully managing interior temperature, light, nutrients, and relative humidity (RH). Many greenhouse studies have been conducted on the impacts of various temperatures, light, nutrients regimes inputs designed to optimize the productivity of horticultural crops (Palencia et al., 2013; Choi et al., 2015; Lee et al., 2015; Choi et al., 2016; Park et al., 2018), but research on the effects of RH are relatively scarce. Low RH (< 40%) during daylight hours from November to March in Korea is especially problematic for producing horticultural crops due to dryness damage of leaves with drying inside a greenhouse (Choi et al., 2019).
A number of photosynthetic and chlorophyll fluorescence measurements have been used to indicate whether greenhouse plants are thriving or stressed (Choi et al., 2016; Choi and Kang, 2019). For example, leaf health (and by extension, productivity) of horticultural crops are often evaluated by measuring photosynthetic rate, stomatal conductance, and transpiration rate (Chrysargyris et al., 2019; Khaleghnezhad et al., 2019). In particular, the transpiration rate of leaves is sometimes used to indicate the environmental adaptability of leaves (Shuaishuai et al., 2018) and the formazan pigmentation of roots is expressed to indicate (indirectly) root activity of crops under a given environmental regime (Kang et al., 2010). Likewise, the initial chlorophyll fluorescence curve part of 0 ‑ 30 ms (OJIP) test of chlorophyll fluorescence helps researchers understand and characterize energy transfer between photosystem II (PSII) units, which can be used to describe all pathways of this communication under a variety of environmental conditions (Kalaji and Guo, 2008). For example, the OJIP test can be used to evaluate stress in horticultural crops by examining parameters related to electron transfer in PSII, such as an average absorbed photon flux per cross section of the sample (ABS/CS), an energy flux trapped by PSII of a photosynthesizing sample cross section (TRo/CS), a rate of electron transported by PSII of a photosynthesizing sample cross section (ETo/CS), and an energy flux not intercepted by photosynthesizing sample cross section (DIo/CS) (Oh et al., 2014; Zushi and Matsuzoe, 2017).
Strawberry (Fragaria × ananassa Duch.) is one of the most widely consumed fresh fruits in the world. Strawberry is also an economically valuable horticultural crop in Korea, where, in 2016, 5,978 ha were devoted to cultivating strawberry. Production averaged 191,218 kg/10a, with an annual revenue of 1.3 billion dollars in 2016 (MAFRA, 2017; Choi and Kang, 2019). However, the production of strawberries in the main producing provinces on Korea declined in 2016 relative to 2015 (MAFRA, 2016, 2017). It is believed that low RH in strawberry-growing greenhouses was one of many reasons for the decline according to unpublished information from Rural Development Administration of Korea. Although most strawberry growers in Korea diligently manage temperature and light conditions in greenhouses from early winter to early spring, few attempt to manage humidity levels in greenhouses.
Because RH is so crucial to the productivity of strawberries grown in greenhouses, our objective was to quantify the effects that humidity has on strawberry productivity during early winter and early spring when RH is usually low. To evaluate the impact of RH in growth chamber which were controlled an environment conditions, we measured several photo-physiological attributes, such as photosynthetic rate and photosynthetic efficiency (using the OJIP test of chlorophyll fluorescence), in two strawberry varieties cultivated in Korea.
Materials and Methods
Plant Materials and Cultivation
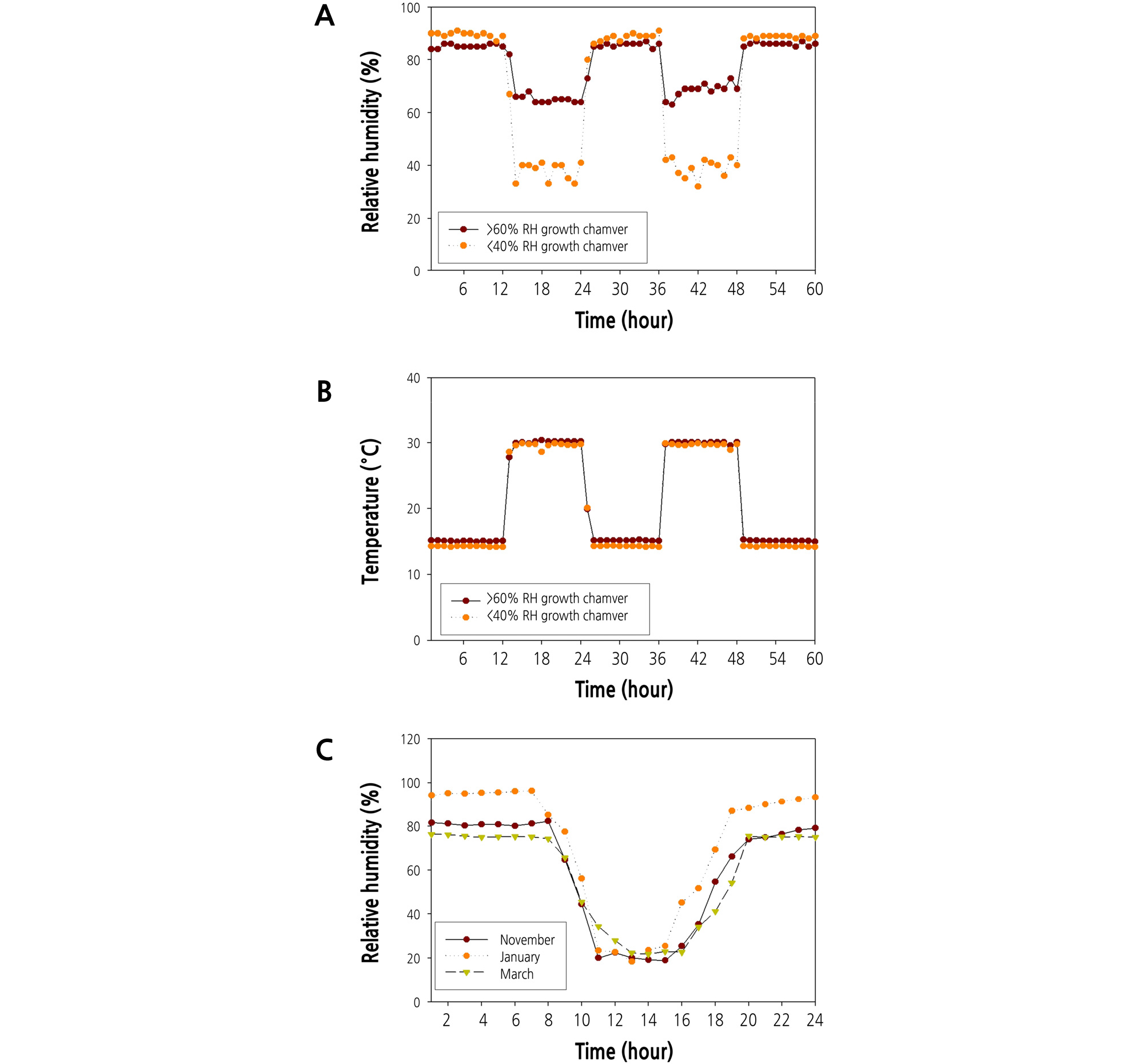
We planted strawberry cultivars (Fragaria × ananassa Duch. cv. ‘Arihyang’ and “Keumsil”) in 500 mL pot filled with a commercial soil medium (Tosille Medium, Shinan Grow Co., Jinju, Korea). We grew all experimental strawberry plants in the glass greenhouse of Kongju national University, Korea (36°67'N, 127°86'E) from September 20 to November 19, 2018. On November 20, pots of strawberries were moved into two different growth chambers (DF-95G-1248M, Duri science Inc., Bucheon, Korea), one chamber with a daytime RH of < 40% and the other with RH > 60% (Fig. 1A). The temperature of both growth chambers was maintained at 30°C during the day and 15°C at night (Fig. 1B). Artificial lighting in the growth chambers was provided by a mixture of LEDs and metal halide lamps that irradiated the canopy of strawberry plants with 250 µmol·m-2·s-1 of light intensity during the day. Plants were supplied with 200 mL of strawberry nutrient solution [macro-element (N: P: K: Ca: Mg: S = 12.5: 3.0: 5.5: 6.5: 2.5: 3.0 me·L-1), micro-element (Fe: B: Mn: Zn: Cu: Mo = 1.12: 0.27: 0.55: 0.46: 0.05: 0.05 mg·L-1), electrical conductivity (EC) = 1.0 dS·m-1, hydrogen ion concentration (pH) = 5.5 ‑ 6.0, using the Research Station for Floriculture and Glasshouse Vegetables (PBG) nutrient solution] per day from November 20 to December 20, 2018.
Analysis of Photosynthetic Rates and Chlorophyll Fluorescence
We measured photosynthetic rates and chlorophyll fluorescence OJIP transients in 20 to 30-day-old strawberry leaves (n = 10 leaves per treatment). On December 5 and December 20, we used the portable photosynthesis system (LI-6800, Licor, NE, USA) to measure photosynthetic rates, stomatal conductance, and transpiration rates of strawberry leaves in the two growth chambers, the photosynthesis system chamber adjusted to 1,000 µmol·m-2·s-1 of light (LED) intensity. After 30 minutes of allowing strawberry leaves to adapt to darkness, we measured chlorophyll fluorescence in leaves with a portable chlorophyll fluorescence instrument (FluorPen FC 100, Photon Systems Instruments, Drasov, Czech Republic). Table 1 provides a detailed description of all chlorophyll fluorescence OJIP transients.
Table 1. Summary of the OJIP-test parameters using data extracted from the fast fluorescence transient
Analysis of Root Activity
We measured root activity in strawberries at 470 nm using an UV-visible spectrophotometer (Evolution 300, Thermo Co., CA, USA) following the method described by Kang et al. (2010). After washing fresh root samples with running water, we cut the roots to 2-cm lengths, mixed them with other root cuttings of the treatment to attain 500 mg of root weight per treatment, and placed the mixture in a glass test tube. To each sample tube, we added 10 mL of a mixture composed of TTC solution, 0.1 M sodium phosphate buffer solution, and distilled water at a ratio of 1:4:5. After allowing air bubbles to dissipate over a 10-minute period, we continued the reaction in the dark for two hours in a constant temperature bath maintained at 30°C. We then added 2 mL of 2N H2SO4 to the sample tubes, vortexed them, and then rinsed the samples with running water. We then added 10 mL of ethyl acetate and 1 g of crushed quartz glass to the reacted root samples and filtered the mixture through a filter paper (110 mm) (No. 2 filter paper, Advantec Mfs Inc., CA, USA) to extract formazan. The formula for calculating the activity of roots using formazan is the amount of formazan (mg) divided by root weight (g) times reaction time (h).
Experimental Design and Statistical Analysis
This study was a randomized block design conducted in two growth chambers (n = 10 plants/treatment) and was two repeats. We separated each treatment into four blocks (two RH conditions × two strawberry cultivars); we measured the photosynthetic rate, transpiration rate, stomatal conductance, root activity, and chlorophyll fluorescence OJIP transients for all plants in each block. The effects of RH and cultivar were analyzed with a two-way analysis of variance (ANOVA). In addition, experimental data were subjected to ANOVA and were analyzed with Duncan’s multiple range test (p = 0.05) using SAS (SAS Institute Inc., NC, USA).
Results
RH in Korea During the Cold Growing Season
Changes in RH and temperature over time by the growth chamber (< 40% and > 60%), are shown in Fig. 1A and 1B. Artificial conditions in growth chambers were designed to mimic extremes of RH conditions from November to March in a greenhouse at Kongju National University (Fig. 1C). During the cold growing season, the greenhouse humidity in Korea declines to about 20% RH during the day.
In our experiment, both strawberry cultivars grown under < 40% RH conditions for 30 days were more withered than strawberries grown under > 60% RH conditions (Fig. 2).
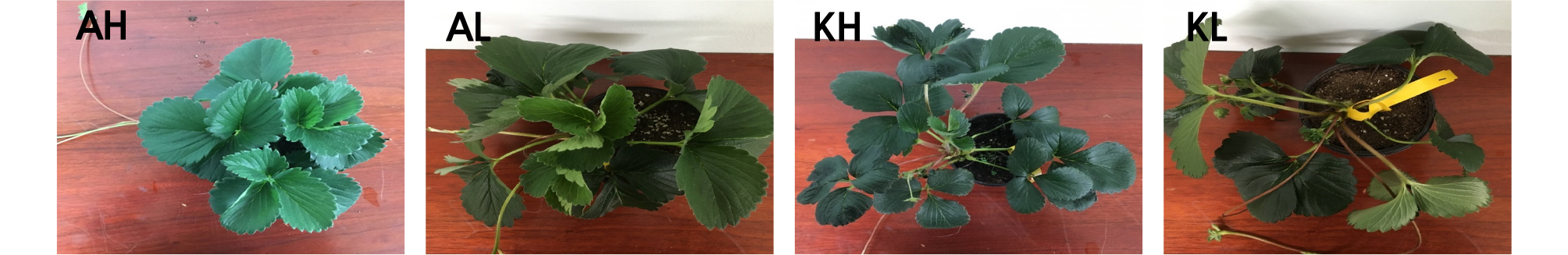
Fig. 2.
Two strawberry cultivars grown under different relative humidity (RH) regimes over a 30-day period. Panels: AH [‘Arihyang’ cultivar grown under high humidity (> 60% RH) conditions], AL [‘Arihyang’ cultivar grown under low humidity (< 40% RH) conditions], KH (‘Keumsil’ cultivar grown under high humidity conditions), and KL (‘Keumsil’ cultivar grown under low humidity conditions).
Photosynthesis Parameters of Leaves of Two Strawberry Cultivars Grown Under Different RH Conditions
After 15 days of humidity treatment (< 40% and > 60% RH), transpiration rates of strawberry leaves did not show any statistics significant difference, but there was a significant difference in transpiration rates relative to the type of cultivar in two-way ANOVA (about 17 mmol H2O·m-2·s-1 of ‘Arihyang’ showed a higher rate than about 13 mmol H2O·m-2·s-1 of ‘Keumsil’). After 30 days of humidity treatment, we found no significant difference in transpiration rates relative to cultivar type, but the longer drying period during the daytime, leaves of strawberries gown under < 40% RH were significantly less the transpiration rates than that of strawberries in > 60% RH condition (Fig. 3). We judged these results to be indicated that, as shown in Fig. 2, transpiration rate of strawberry plants grown at < 40% RH decreased drastically as a stoma control mechanism to prevent drought stress due to low humidity condition related with increasing vapor pressure deficit (VPD) value. Photosynthetic rate and stomatal conductance compared to the transpiration rate of strawberry plants analyzed after 15 days of humidity treatment, these of strawberry leaves decreased significantly at < 40% RH. Especially, the ‘Keumsil’ cultivar grown under < 40% RH was on the lowest than any other treatment. In result of two-way ANOVA analysis, both photosynthetic rate and stomatal conductance of leaves responded significantly to cultivar type and RH after 15 days of humidity treatment. In contrast, similar to transpiration rates, the significant difference of photosynthetic rate and stomatal conductance occurred only in response to RH after 30 days of humidity treatment. The response to RH was greater for transpiration rate, photosynthetic rate, and stomatal conductance than the response to the cultivar type as longer the elapsed treatment period. After 30 days of RH treatment, the transpiration rate of the two strawberry cultivars grown under < 40% RH condition was less than when grown under > 60% RH condition.
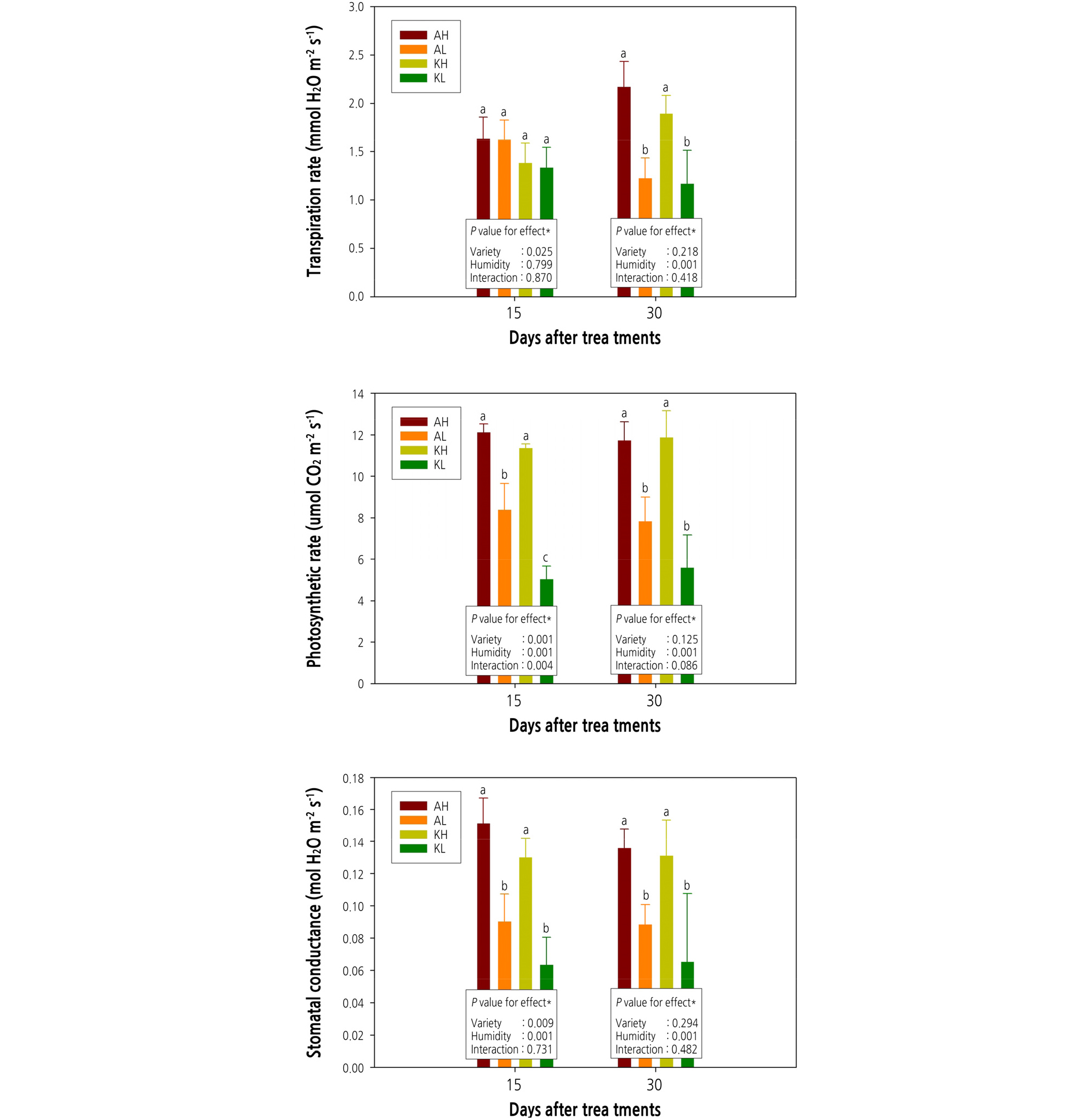
Fig. 3.
Transpiration rate, photosynthetic rate, and stomatal conductance of two strawberry cultivars grown under different two relative humidity conditions. Panels: AH [‘Arihyang’ cultivar grown under high humidity (> 60% RH) conditions], AL [‘Arihyang’ cultivar grown under low humidity (< 40% RH) conditions], KH (‘Keumsil’ cultivar grown under high humidity conditions), and KL (‘Keumsil’ cultivar grown under low humidity conditions). P values determined with two-way ANOVA. Boxplots marked with letters that differ from other boxplots have means that differ significantly from the other boxplots, based on Duncan’s multiple range test (p < 0.05).
Chlorophyll Fluorescence OJIP Transient Parameters of Leaves of Two Strawberry Cultivars Grown Under Different Relative Humidity Conditions
The change in chlorophyll fluorescence OJIP curve and the intensity change in each OJIP step values are shown in Fig. 4. It was no statistically significant difference in OJIP transient values for leaves among cultivars or humidity that could be attributed to a stressed leaves. However, in the case of ‘Arihyang’, the OJIP transient curve pattern was different depending on the RH treatment (A and B in Fig. 4).
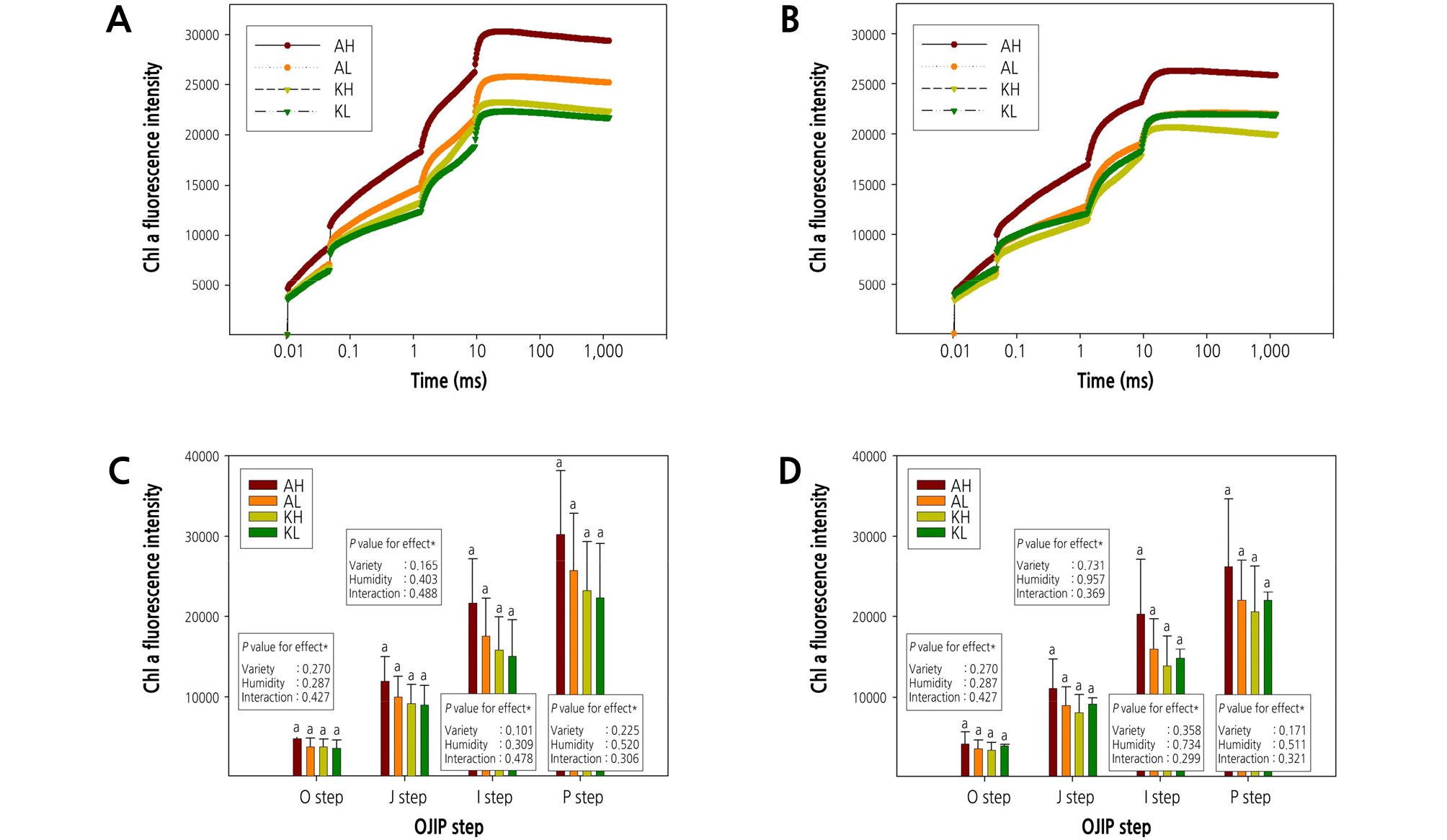
Fig. 4.
Chlorophyll a fluorescence OJIP transient curves for two strawberry cultivars grown under different two relative humidity conditions. A and C compare measurements obtained after 15 of humidity treatment (Dec. 05). B and D compare measurements obtained after 30 days of humidity treatment (Dec. 20). Panels: AH [‘Arihyang’ cultivar grown under high humidity (> 60% RH) conditions], AL [‘Arihyang’ cultivar grown under low humidity (< 40% RH) conditions], KH (‘Keumsil’ cultivar grown under high humidity conditions), and KL (‘Keumsil’ cultivar grown under low humidity conditions). P values determined with two-way ANOVA. Boxplots marked with letters that differ from other boxplots have means that differ significantly from the other boxplots, based on Duncan’s multiple range test (p < 0.05).
On the other hand, we identified a significant difference between cultivars and RH on the various values, which were calculated with chlorophyll fluorescence OJIP transient curves (Tables 2 and 3). The Fv/Fm value is defined maximum quantum efficiency of PSII photochemistry and is the most common indicator of chlorophyll fluorescence transient on plant leaves under stress conditions in general. In case of Fv/Fm value in our study, there was no difference according to the humidity treatment, but the difference by cultivars was significant after 15 and 30 days of RH treatment, and it was no significant difference in the interaction between cultivar and RH condition. Ss value representing reflecting multiple turnover of QA reductions was significantly higher at < 40% RH than that of > 60% RH. In contrast to Fv/Fm, Ss value was no difference between cultivars, but the difference in RH was statistics significant. The relative variable chlorophyll fluorescence value at a logarithmic time scale, such as Vj (after 2millisec) and Vi (after 30millisec) were significantly different in both cultivars after 30 days of RH treatment. In ‘Arihyang’, especially Vi value was a significant difference according to RH condition. In Vj and Vi values, ‘Arihyang’ was higher than ‘Keumsil’, and ‘Arihyang’ was significantly higher when the humidity in growth chamber was higher. Mo, this parameter reflects the rate at which PSII reaction centers are closed and it was not affected by cultivar and RH condition. In our result of this study, ‘Arihyang’ was more affected by RH condition than ‘Keumsil’ (Table 2). DIo/RC, this value reflects the energy flux not intercepted by a reaction center, which is no significant difference in our study treated with RH and cultivar. The ABS/RC, TRo/RC, and ETo/RC parameters, related to energy flux per PSII reaction center, of both cultivars, responded strongly to RH. In particular, energy flux parameters for leaves grown under < 40% RH condition were significantly lower than leaves grown under > 60% RH condition (Table 3).
Table 2. Chlorophyll fluorescence parameters in the leaves of two strawberry (Fragaria × ananassa) cultivars grown under different RH conditions
yP values were determined by two-way ANOVA.
xValues followed by different lower case letters within a column are significantly different (Duncan's multiple range test, p < 0.05, n = 10).
Table 3. Energy flux parameters per reaction center in the leaves of two strawberry (Fragaria × ananassa) cultivars grown under different RH conditions
Root Activity of Two Strawberry Cultivars Grown Under Different Relative Humidity Conditions
We found that formazan content in strawberry roots differed significantly relative to cultivar type, humidity, and the interaction of cultivar and humidity (Fig. 5). Roots of the ‘Arihyang’ cultivar grown under > 60% RH possessed a higher formazan content than the roots of the ‘Keumsil’ cultivar and when grown under < 40% RH. Taken together with our results on the relationships between RH and stomatal conductivity and transpiration rates, it was that high root activity of the two strawberry cultivars is similar to the definitive response of leaves grown under > 60% RH condition (Figs. 2, 3, and 5).
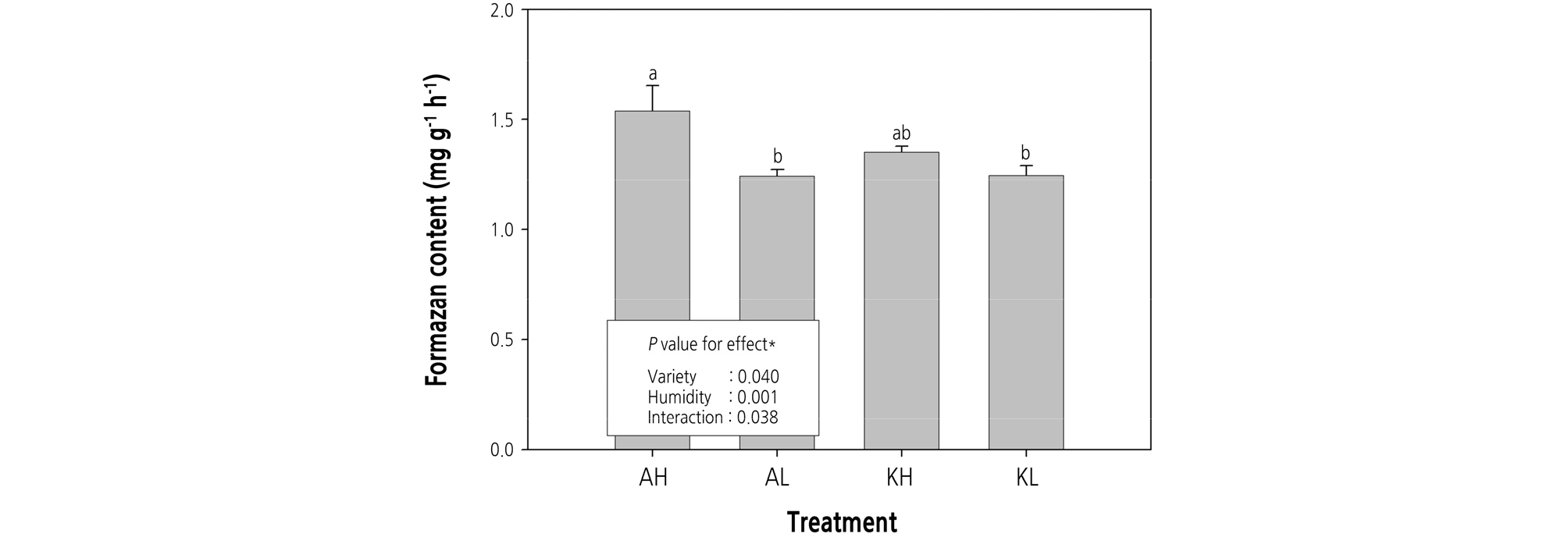
Fig. 5.
Root activity of two strawberry cultivars grown under different two relative humidity conditions. Panels: AH [‘Arihyang’ cultivar grown under high humidity (> 60% RH) conditions], AL [‘Arihyang’ cultivar grown under low humidity (< 40% RH) conditions], KH (‘Keumsil’ cultivar grown under high humidity conditions), and KL (‘Keumsil’ cultivar grown under low humidity conditions). P values determined with two-way ANOVA. Boxplots marked with letters that differ from other boxplots have means that differ significantly from the other boxplots, based on Duncan’s multiple range test (p < 0.05).
Discussion
RH is a very important environmental control factor to grow horticultural crops that control the environment in greenhouses than horticultural crops grown in open field. RH in a greenhouse greatly influences the growth rate of horticultural crops growing inside, (Mortensen, 1986) and so, low RH in greenhouses growing tomatoes is believed to reduce early yield (Barker, 2015). In our experiments, the photosynthetic and chlorophyll fluorescence related parameter values in the leaves as well as the root activity of strawberry plants were negatively affected when these grown at low RH condition (Tables 2 and 3, Figs. 3 and 5). Mortensen (2000) reported that the high humidity enhanced the plant dry weight of poinsettia and kalanchoe grown in a greenhouse, and Harel et al. (2014) reported that the increased RH from 50% to 70% during day time improved the pollen grain’s viability of tomato in commercial cultivation. Both results agree with our study showing that high RH conditions have a positive effect on photosynthetic rate, transpiration rate, and stomatal conductance of strawberry leaves. Therefore, we speculate that low RH detrimentally affects strawberry leaf productivity (regardless of all cultivars) over time.
Analytical methods of photo-physiology by using photosynthetic rate of horticultural crops are used in many greenhouse studies because they are closely related to substantial productivity gains and it is widely used to predict the productivity improvement of horticultural crops grown under various environmental conditions in a greenhouse (Deyton et al., 1991; Kadir et al., 2006; Choi et al., 2014; Hidaka et al., 2016; Ayad et al., 2018). Especially, Choi et al. (2016) also reported that a positive correlation exists between strawberry fruit production and photosynthetic parameters, such as photosynthetic rate, transpiration rate, stomatal conductivity, and values of chlorophyll fluorescence transients. Based on these findings, strawberry yields in greenhouses during dry-cold weather (early winter to early spring) can be kept stable if a relatively high humidity can be maintained inside the greenhouses mainly because high RH enhances root activity and photosynthetic rates than low RH.
Some studies related chlorophyll fluorescence have shown that horticultural crops significantly differ in OJIP steps relative to the degree of stress and that such plants exhibit remarkable deformation of OJIP curves form under stressed conditions (Oh et al., 2014; Zushi and Matsuzoe, 2017). Unlike some previous studies, the shape of the OJIP curve of strawberry leaves did not change much in our experiment related with RH condition in the growth chamber. However, the pattern of downward trend OJIP curves of ‘Arihyang’ strawberry leaves grown at low RH was similar to the pattern of wheat leaves under drought condition in report of Kalaji et al. (2016). In this OJIP curve pattern, we confirmed that ‘Arihyang’ is more sensitive to low RH condition than ‘Geumsil’. Also, Maxwell and Johnson (2000) postulated that the maximum quantum efficiency of PSII photochemistry (Fv/Fm) ratio value in the range of 0.79 to 0.84 is optimal for many plant species. The value of Fv/Fm in our study fell within that range in all our treatments, meaning that Fv/Fm ratios would be difficult to use as an indicator of stress in cultivation of horticultural crops, except perhaps for plants grown under extremely stressful conditions. In addition, generally known OJIP curve shapes would be difficult to identify physiological responses to horticultural crops except grown under extreme environmental stresses conditions. On the other hand, as a result of our study of confirming the individual parameter values of the OJIP curve, there was a statistically significant difference by the humidity treatments. The Vj and Vi parameters, calculated to chlorophyll fluorescence OJIP curves, differed significantly between the two cultivars we tested. Whereas the Ss parameters, related to turnover of QA (an electron transfer material) reduction, were significantly related to RH (Table 2). Also, the parameter values associated with the energy flux per reaction center of chlorophyll fluorescence in the leaves of strawberry during photosynthesis reaction were found to have a significant relationship with change of the RH conditions (Table 3). We speculate that the activity of electron transfers on the photosystem is strongly affected by RH. Previous studies of various crops grown under drought conditions (under low humidity) have suggested the potential efficacy of using the OJIP test values to identify crops under drought stress (Rapacz et al., 2019; Wang et al., 2019; Zhou et al., 2019). Therefore, based on our results and those of others, we believe that the OJIP transient test could be used effectively to monitor the water balance status of horticultural crops grown under a variety of greenhouse environments.
In aspect of vapor pressure deficit (VPD) relative to humidity inside a greenhouse, Kroggel and Kubota (2016) reported that the main cause of reduced yield of a greenhouse strawberry is tipburn, which is associated with high VPD value and low RH. Also, they explained that strawberry leaves grown under high VPD condition were reduced calcium supply to the meristematic tissues as well as were limited the transpiration rate. These low RH conditions not only cause primary damage such as plant photosynthesis inhibition, but also secondary damage such as tipburn of leaves. These findings were similar to our study in which the rate of transpiration rate of strawberry leaves was reduced at low RH. Therefore, RH control is a very important factor when cultivation of a greenhouse strawberry.
As the two conclusions from our study, first, we suggest that in addition to photosynthesis-related parameters, chlorophyll fluorescence parameter values such as energy flux per PSII reaction center, Vj, Vi, and Ss of OJIP can be applied to monitoring the productivity of horticultural crops grown under various environmental conditions in a greenhouse. Second, most of the strawberry farmers in Korea currently overlook the RH-related environmental control in a greenhouse, which reduced the photosynthesis efficiency of strawberry plants and cause serious production decrease. Therefore, increasing of strawberry yield due to controlling proper RH in a greenhouse is very important for stable supply of strawberry fruit for domestic and export.