Introduction
Materials and Methods
Plant Materials
Experimental Design and Treatments
Application of Chemical Mutagens
Rate of Mutation
Phenotypic Evaluation
Evaluation of Stomata Characteristics
Ploidy Analysis
Data Collection and Analysis
Results
Rate of Mutation
Echeveria ‘Brave’
E. ‘Viyant’ (E. cuspidata var. Zaragozae × E. lauii)
Echeveria ‘Snow Bunny’ (E. elegans × E. lauii)
Stomata and Ploidy Analysis
Discussion
Introduction
Succulents, which form a recognizable part of the terrestrial ecosystem, are fascinating plants that have captured the curiosity of people because of their storage capability, which allows them to maintain their quality despite long periods of drought (Males, 2016). Ogburn and Edwards (2010) reported that based on the structure of their tissues, succulents provide morphological and phylogenetic diversity. Succulent plants photosynthesize via Crassulacean acid metabolism, which allows higher uptake of CO2, and the plants adapt easily to drought conditions (Hanscom and Ting, 1978; Taiz and Zeiger, 2010), making them great indoor air-purifying pot plants. Hence, succulents are popular house plants because they can improve air quality and absorb toxic chemicals from common household products. They are beneficial for multiple age groups who suffer from asthma and other contaminated air-related diseases (Claudio, 2011). Aesthetically, succulent plants are popular indoor and landscape plants because of their unique geometrically shaped leaf structure and formation, which make them aesthetically appealing and can trick onlookers into the assumption that they are faux plants (Baldwin, 2013; Cabahug et al., 2018). Because of their health benefits, aesthetic function, and desirable plant characteristics, there has been an increase in the popularity and demand for succulents, as well as for plants with unique colors and leaf shapes (Cabahug et al., 2018).
In the last decade, mutation breeding for ornamental plants has gained popularity and has been used as a tool to produce new cultivars with unique traits (Datta, 2009). Although there are many available methods of mutation breeding, chemical mutation is one of the most convenient and can induce mutations in plants by creating genetic variation, resulting in desirable traits (Schum, 2003; Mostafa, 2015). Oladosu et al. (2016) detailed several advantages of chemical mutagenesis, including its ease of handling and application without sophisticated equipment or facilities. Major mutagen groups that are commonly used in plant mutagenesis include alkylating agents, azide, hydroxylamine, acridines, and base analogs (Oladosu et al., 2016). Colchicine, an alkaloid derived of Colchicum autumnale, has been consistently used in horticultural plants (Broertjes and Van Harten, 1988; Predieri, 2000; Jain, 2010; Nura et al., 2013), such as with other succulents like aloe vera (Imery and Cequea, 2001), and other potted plants like alocasias (Thao et al., 2003), African violets (Seneviratne and Wijesundara, 2007), cyclamens (Kondo et al., 2009), cymbidium (Hwang et al., 2015) and poinsettias (Pan et al., 2019). The condition of the mutagenic solution, characteristics of the targeted plant, the environment, solution concentration, and treatment duration are important factors that influence the outcome of chemical mutagenesis (Oladosu et al., 2016). However, it has been reported that the frequency and type of mutation outcomes are direct results of the dosage and rate of exposure of the chosen mutagen (Mba, 2013).
The effects of colchicine vary with each crop and even within species. Time of treatment and mutagen concentration are important factors for successful chemical mutagenesis (Schum, 2003; Datta, 2009; FAO, 2017). However, no previous studies have focused on developing new cultivars of ornamental succulent plants using chemical mutagenesis, especially using colchicine. Hence, this study aims to determine the optimum levels of colchicine application and to provide phenotypic data regarding its effects on Echeveria species, including its growth and development.
Materials and Methods
Plant Materials
The selected plant species, with fully expanded leaves, were procured from a succulent nursery in Goyang-si, Gyeonggi-do, Republic of Korea. The selected Echeveria species for the study were E. ‘Brave’, E. ‘Viyant’ (E. cuspidata var. Zaragozae × E. lauii), and E. ‘Snow Bunny’ (E. elegans × E. lauii). Plants were transported to Sahmyook University, Seoul, Republic of Korea. Leaves from the three lower whorls of the plant were removed from the mother plant to create uniform size and mature leaf-cutting propagules. Leaf cuttings were randomly selected and separated for each mutagen treatment.
Experimental Design and Treatments
The study was conducted using a 5 × 4 factorial arrangement using a completely randomized design. Five concentrations (0.2, 0.4, 0.6, 0.8, and 1.0%) and four treatment durations (3, 6, 9, and 12 h) were used in the study. Each treatment combination was replicated five times with 10 leaf cuttings per replication for a total of 50 leaves per treatment combination. Untreated leaf cuttings that were directly planted after detachment served as control. Previous literature was reviewed and served as the basis for determining the concentration levels and treatment durations used in the study.
Application of Chemical Mutagens
The chemical mutagens were placed in containers in which leaf cuttings were placed upright to submerge the growing point of the leaf cuttings, such that the expected point of absorption was exposed. These treatment containers were then placed in a darkened fume hood to facilitate aeration and avoid exposure to light during the treatment. After the designated treatment time, the leaf cuttings were carefully removed from the treatment trays and planted in a nursery.
Rate of Mutation
The rate of survival and number of active Echeveria leaf cuttings that successfully produced shoots and roots were counted at 12 weeks after treatment. The putative mutants were separated, planted into individual plants, and subjected to phenotypic evaluation. To determine the rate of mutation, the number of mutants over the number of successfully developed plants was multiplied by 100.
Phenotypic Evaluation
Prior to evaluation of the M1 generation’s phenotype, an LD50 study was conducted (Cabahug et al., 2020). Based on forward genetics methods, mutant succulents were screened using phenotypic categories in comparison to those of the control, which include leaf morphology and chimeras (Wu et al., 2005; Taylor, 2017). Phenotypic data were collected from successfully mutated succulents. These were divided into two categories: a.) plant parameters, which included plant height and diameter, and leaf length, width, and thickness, as well as CIELAB color (Spectrophotometer CM-2600d, Konica Minolta Inc., Japan); and b.) plant structures, which described the shape, edge, and apex, as published in the Manual of Leaf Architecture (Ash et al., 1999).
Evaluation of Stomata Characteristics
Based on the mutant rate, three treatments were chosen for stomata evaluation. Mutant plants were randomly selected within each treatment and leaves were taken from the base. The nail varnish technique (Gitz and Baker, 2009) was used for evaluating stomata size and density. The samples were studied under a light microscope (Olympus BX53F, Japan) at 40x and 80x magnification. To determine the density of stomata, counts were taken thrice per leaf at random locations across the surface. The stomata size was measured using Image J (v 1.52a, USA).
Ploidy Analysis
To determine genetic variations with colchicine-treated succulents, a ploidy level analysis was done using flow cytometry (FCM). Succulent leaves from selected mutant plants were chopped with a razor blade in 500 mL nuclei extraction buffer (Partech, GmbH, Münster, Germany) and incubated for 10 seconds. The suspension was strained through 30-µm nylon mesh and stained with 2 mL of DAPI containing staining buffer (Partech, GmbH, Münster, Germany). The total DNA was measured for each nucleus with a flow cytometry system (CyFlow, ploidy analyzer, Partech, GmbH, Münster, Germany).
Data Collection and Analysis
Data were collected 12 months after treatment (MAT). Results were subjected to standard descriptive statistics and an analysis of variance (ANOVA) using SPSS (Version 20, IBM Statistics) and Duncan’s multiple range test to compare means.
Results
After identifying the mutant plants from 20 colchicine treatment conditions per species, the results suggested that some treatments failed to produce plants and/or mutant plants. Results are given by species below.
Rate of Mutation
Table 1 shows the survival rate at 12 weeks after treatment. E. ‘Brave’ mutants were observed from 0.2% at all treatment durations (8.82 ‑ 12.50%). At 0.4%, leaf cuttings successfully produced mutants (12.50 ‑ 25.00%); however, when treated at 12 h, there were no putative mutants. For higher concentrations (0.6%, 0.8%, and 1.00%), the leaf cuttings produced mutants when treated at 3 h. Those treated with 0.8% and 1.00% also produced 1 or 2 mutant plants. For E. ‘Viyant’ species, mutants were observed from 0.2% at 6 h (4.88%) and 9 h (4.65%). At 0.4 ‑ 1.00%, similar trends were observed where mutants were taken at these concentrations at 3, 6, and 12 h. Putative mutants were observed from only those at 9 and 12 h from all the concentrations of E. ‘Snow bunny’ despite higher survival rates on lower treatment duration.
Table 1.
Survival and mutant rate (%) of Echeveria species induced with colchicine (n = 50)
Echeveria ‘Brave’
Fifteen out of the 20 treatments successfully developed mutant succulent plants (Table 2). E. ‘Brave’ species treated for 3 h developed mutant plants at all five concentration levels, but as dipping time increased, fewer treatments were able to produce mutant plants. However, the highest number of treatments with mutant plants was observed for those exposed to 0.20% and 0.80% concentration. Table 3 shows the CIELAB color reading for E. ‘Brave,’ indicating that a* and b* were affected by colchicine treatments (p < 0.01). Compared to that of the control, mutated plants had darker tones with the majority of treated species described as greyed-green.
Table 2.
Plant parameters of mutated Echeveria 'Brave' treated with colchicine with different concentrations and dipping times at 12 months after treatment (MAT)
Table 3.
Color reading and equivalent color group based on the Royal Horticultural Society (RHS) color scheme for mutated Echeveria 'Brave' species
Untreated E. ‘Brave’ was described as having a cuneate leaf shape and an acute leaf apex; however, these characteristics changed in the colchicine-mutated plants, which were more compact and had an obtuse or obovate leaf shape and apex. This indicated a wider leaf apex but shorter leaves (Fig. 1A).
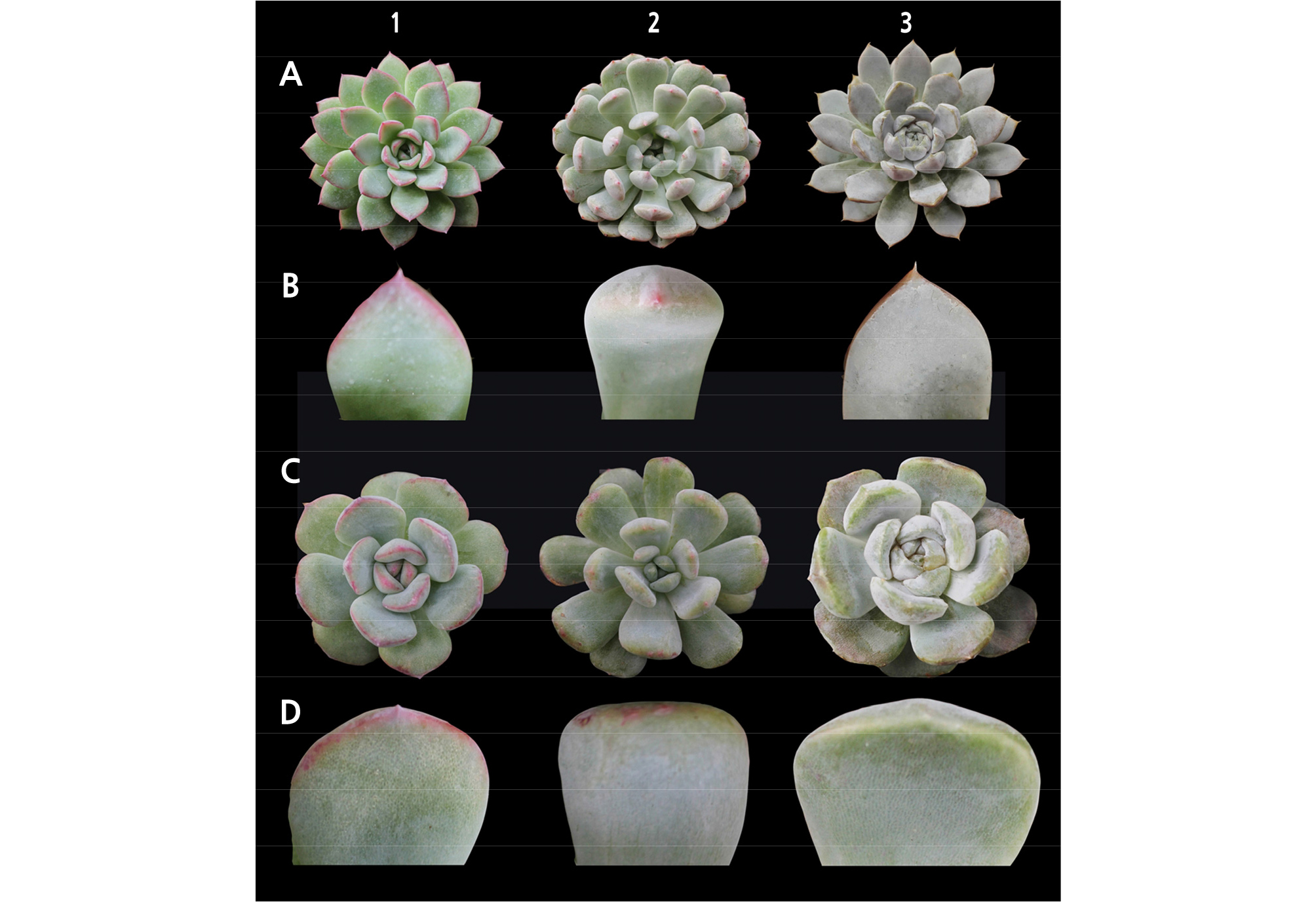
Fig. 1.
Sample comparison of the control (A, whole plant; B, leaf apex shape) and the most frequently observed phenotypic characteristics for mutant plants treated with colchicine (C, whole plant; D, leaf apex shape) of selected Echeveria species: 1, E. ‘Brave’; 2, E. ‘Viyant’ (E. cuspidata var. Zaragozae × E. lauii); and 3, E. ‘Snow Bunny’ (E. elegans × E. lauii).
E. ‘Viyant’ (E. cuspidata var. Zaragozae × E. lauii)
Only 14 out of the 20 treatments produced mutants (Table 4). There were three treatments in each concentration from 0.40% to 1.00%; for those treated with 0.20%, only two treatments produced mutated plants. Among dipping durations, treating succulent leaves for 6 h produced mutated plants in five treatments (Table 4: A, D, G, J, and M). This was followed by those exposed for 3 h (Table 4: C, F, I, and L) and 12 h (Table 4: E, H, K, and N), for which four treatments produced mutants, while 9 h of treatment had the least, with only one mutant (Table 4: B). The control had the thinnest (5.92 mm) but longest leaves (48.31 mm). The outcome of these combined leaf characteristics makes a plant that exhibits compactness.
Table 4.
Plant parameters of mutated Echeveria 'Viyant' (E. cuspidata 'Zaragozae' × E. lauii) treated with colchicine with different concentrations and dipping times at 12 MAT
Similar to E. ‘Brave,’ the color tones of this species were darker compared to that of the control (Table 5) (p < 0.01). The control was categorized under the grey-brown color group. However, those treated with colchicine were in the grey-brown to brown color group range.
Table 5.
Color reading and equivalent color group based on the Royal Horticultural Society (RHS) color scheme for mutated Echeveria 'Viyant' (E. cuspidata 'Zaragozae' × E. lauii) species
The leaf shape and apex of E. ‘Viyant’ was somehow altered by the mutagen. From the original linear leaf shape, mutated succulents produced cuneate leaves. Likewise, the leaf apex of the mutants had a wider apex and less prominent bristle point compared to that of the control (Fig. 1B).
Echeveria ‘Snow Bunny’ (E. elegans × E. lauii)
Colchicine-treated plants had 10 treatments that successfully produced mutant species (Table 6). There were no mutants for the 3 and 6 h dipping duration. Compared to that of the control, mutated E. ‘Snow Bunny’ was taller and larger. However, leaf measurements showed the opposite trend wherein the control had a wider and longer leaf.
Table 6.
Plant parameters of mutated Echeveria 'Snow Bunny' (E. elegans × E. lauii) treated with colchicine with different concentrations and dipping times at 12 MAT
For the color values (Table 7), E. ‘Snow Bunny’ mutated plants were lighter compared to the control (p < 0.01). The control was categorized under the greyed-green group. The colchicine-induced succulents were categorized in grey-green (Table 7: A, B, C, H, and J), yellow-green (Table 7: D, E, F, and I), and grey (Table 7: G) groups. Evident leaf structure changed for E. ‘Snow Bunny.’ The control had a cuneate leaf shape, whereas those treated with mutagens were obtuse.
Table 7.
Color reading and equivalent color group based on the Royal Horticultural Society (RHS) color scheme for mutated Echeveria 'Snow Bunny' (E. elegans × E. lauii) species
Stomata and Ploidy Analysis
The stomata data, size (length), and density are presented on Table 8. Results showed that stomata density and size were significantly affected by the use of colchicine mutagen for all species (Fig. 2). Stomata evaluation of E. ‘Brave’ and E. ‘Snow Bunny’ suggests an increase in the stomata size when applied with a mutagen and when the concentration is increased. On the other hand, stomata density was found to be lower as the mutagen concentration increased. In the case of E. ‘Viyant’, there were comparable results between mutants and the control in terms of density. In terms of size, the stomata was smaller than those of the control.
Table 8.
Stomata density and size of mutant Echeveria species treated with colchicine
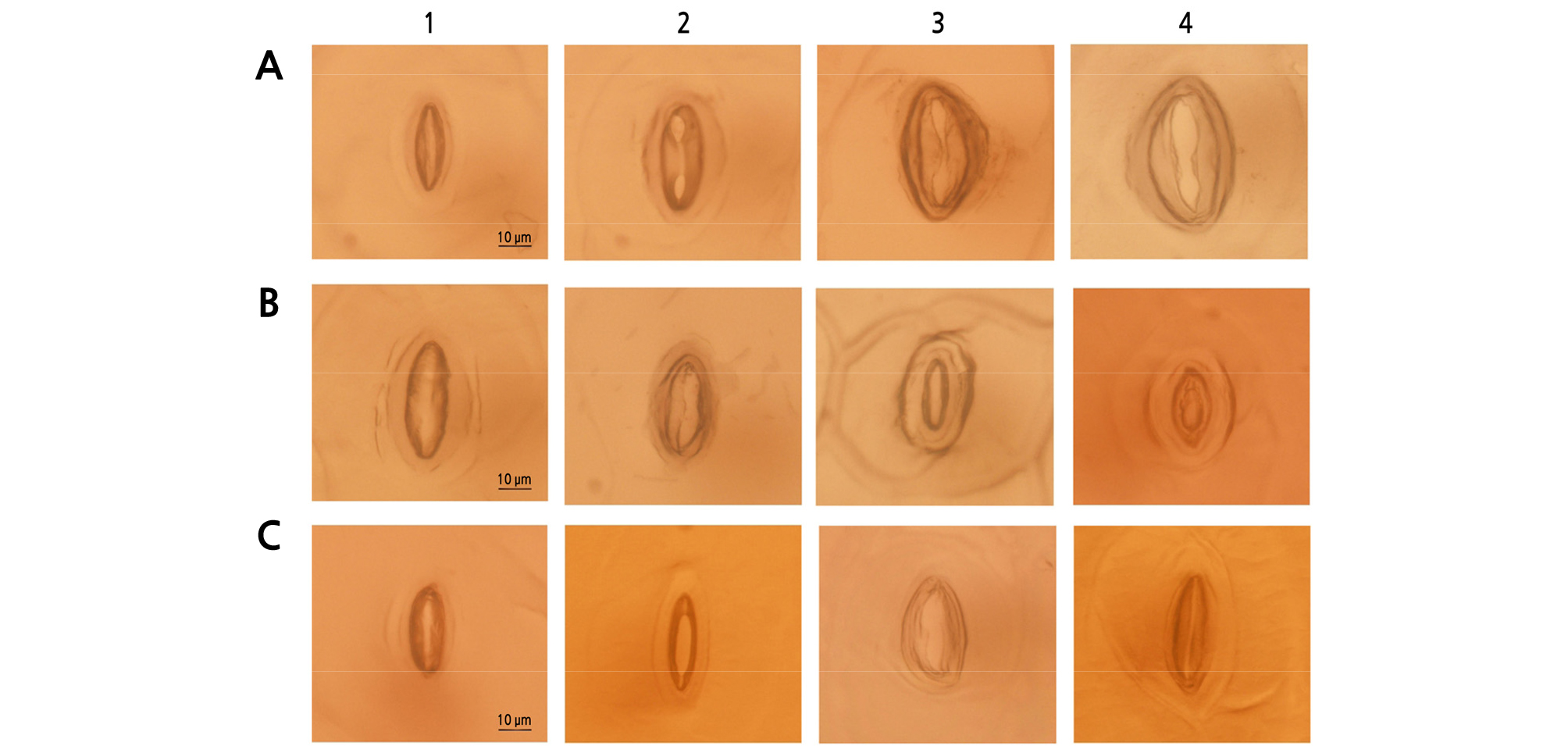
Fig. 2.
Comparison of stomata characteristics of Echeveria species: A, E. ‘Brave’; B, E. ‘Viyant’ (E. cuspidata var. zaragozae × E. lauii); and C, E. ‘Snow Bunny’ (E. elegans × E. lauii), which were treated with: 1, Control; A2, 0.2% + 3 h; A3, 0.4% + 3 h; A4, 1.0% + 3 h; B2, 0.4% + 12 h; B3, 0.8% + 3 h; B4, 0.8% + 12 h; and C2, 0.2% + 9 h; C3, 0.6% + 12 h; C4, 0.8% + 12 h.
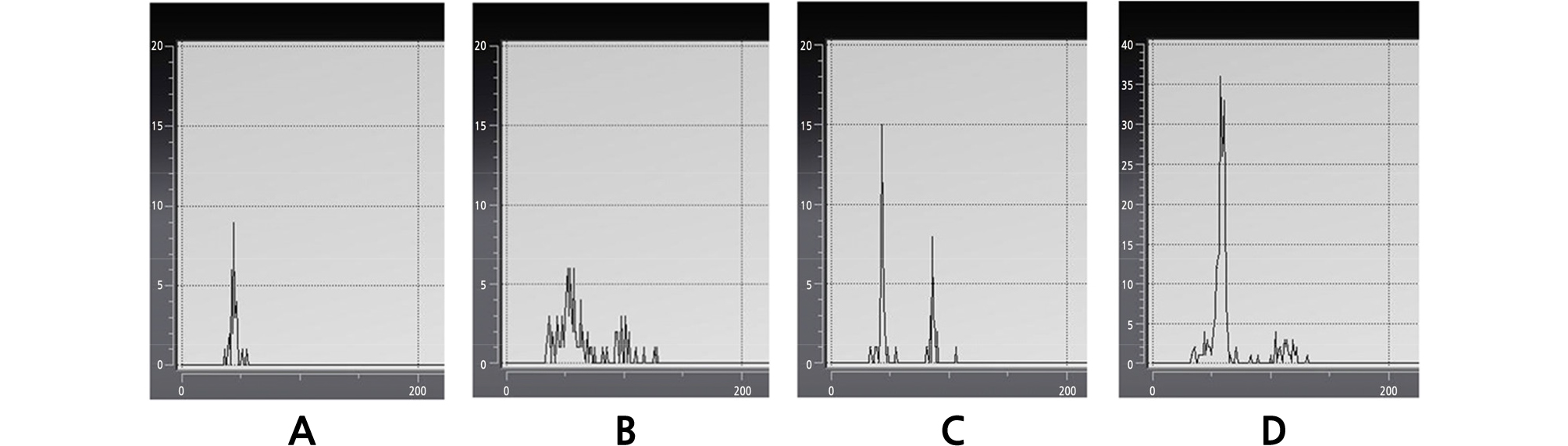
We conducted ploidy analysis to validate the changes in the stomata results. The ploidy levels of mutated succulents obtained from the colchicine treatment were analyzed using FCM (Fig. 3). Diploid control plants showed a peak at channel 50, corresponding to the G1 of ploidy plants (Fig. 3A). The application of colchicine suggests that all three mutated species produced Echeveria of 2x (channel 50) ‑ 4x (Channel 100) complex (Fig. 3B, 3C, and 3D).
Discussion
Manipulating environmental factors can increase ornamental value, these improvements are unstable and easily relapse (Nam et al., 2016; Park et al., 2016; Hoang and Kim, 2018). Hence, the use of mutation induction in ornamental plants is conducted to develop new cultivars which has novel colors and variation to improve ornamental value, especially for ornamentals that are reproduced only through vegetative means (Broertjes and Van Harten, 1988).
There are several advantages of using plant mutagenesis for both the method and the produced mutant. Mutagenesis has been proven to be an appropriate method for ornamental species that have short marketing periods and permanent demand for new fashionable varieties. Thus, varieties are produced faster in mass production (Broertjes et al., 1988; Bhatia, 1991; Kawai and Amano, 1991). Two kinds of traits are observed after inducing mutations: morphological changes and conditional traits. Morphological features include leaf and flower characteristics and growth habit, while conditional traits include changes in photoperiod, flowering duration, and tolerance against biotic and abiotic factors (Schum and Preil, 1998). However, for foliage potted plants like succulents, certain morphological features (e.g. the color and form of leaves, which increase the visual quality) often have a higher value and demand a higher price. Likewise, extraordinary plants with unique features gain more attention compared to common varieties (Sevik et al., 2013).
Various mutagens may provide different effects depending on the plant species and treatment conditions (FAO, 2017). Numerous studies have been conducted to identify the mutagenic efficiency of mutagens used in plant breeding. The efficiency of a mutagen is judged by its ability to produce the maximum desirable changes with the minimum undesirable traits (Kawai, 1975; Girija and Dhanavel, 2009; Begum and Dasgupta, 2010). However, for succulent plants, there have been no studies conducted using chemical mutagens.
We conducted chemical mutagenesis of Echeveria species with colchicine treatment. For many years, colchicine has been favored to induce polyploidy and other inherent changes in horticultural plants (Curry, 1938). Among the selected succulent species, colchicine produced mutant plants for the majority of the treatment combinations (10 ‑ 15 out of 20 treatments). The succulent species E. ‘Snow Bunny’ provided the fewest mutants (Tables 2 and 3), followed by E. ‘Viyant’ (Tables 4 and 5) and E. ‘Brave’ (Tables 6 and 7). Particular succulents naturally possess epicuticular waxes, which produce more or less waxy or powdery coatings (Siems et al., 2014). Studies have suggested that these waxes play important roles in the growth of succulents, including light absorbance and reflection (Mulroy, 1979), photosynthesis (Feakins and Sessions, 2010), and water loss control. Among the species and variants, E. ‘Snow Bunny’ had the most epicuticular waxes in the control. However, during treatment with mutagen, these waxes were significantly reduced. This may be the reason why the survival rates of E. ‘Snow Bunny’ were low and there were no or significantly fewer mutant plants produced.
Colchicine, when applied to plant propagules, produces polyploidy and acts as a mitotic inhibitor (Marzougui et al., 2011). It inhibits the formation of microtubes, which leads to chromosome doubling (Castro et al., 2003). Reports have also shown that there were changes in plant shape and size, for example, in pelargoniums (Jadrná et al., 2010) and marigolds (Sajjad et al., 2013). Similarly, Manzoor et al. (2019) showed that ornamental plants that are polyploids have thicker leaves, more intense coloration of flowers and leaves, and compactness (thicker leaves with stunted growth). In ornamental plants, compact growth is an important trait in production (Van Huylenbroeck et al., 2019). This may be attributed to the “gigas effect”, which increases the size of plant organs among ploidy plants compared to their diploid counterparts. The theory suggests that the growth of organs does not always follow that there is increase of the entire plant size (Sattler et al., 2015). Stebbins (1971) explained that polyploidy plants have a reduced number of cell divisions despite cell enlargement. This explains the morphological changes observed among the majority of Echeveria mutant plants, which had significantly increased leaf thickness but lower plant height and smaller diameter compared to the control.
These changes in growth are parallel to changes in shape and color. According to Curry (1938), colchicine has often been used to cause a ‘burst up’ in which there is a large production of a single species that displays striking changes, such as that of color or leaf shape. Changes in color and leaf shapes have been reported following treatment with mutagens, such as in African violets (Seneviratne and Wijesundara, 2007), pelargonium (Jadrná et al., 2010), and dendrobium (Choopeng et al., 2019).
Flow cytometry analysis further confirmed the morphological changes in Echeveria species. The results suggested that colchicine-induced plants produced 2x ‑ 4x complex mixoploids. These diploid (2x) ‑ tetraploid (4x) chimeras were also observed with colchicine mutagen treatment of aloe vera (Imery and Cequea, 2001), Bacopa (Escandon et al., 2006), Saliva (Kobayashi et al., 2008), Dendrobium (Atichart, 2013), Dracocephalum (Zahedi et al., 2014), and citrus (Nukaya et al., 2020). The treatment was found to increase the ornamental and crop value of the mutants. Results of these studies also suggest morphological (increment and color intensity in leaves, inflorescence, and fruits) and anatomical (stomata size and density) changes. Lin et al. (2011) and Shala and Deng (2018) reported that stomata analysis is a tool for verifying the status of polyploids. Mutated Echeveria had larger stomata size with decreased density compared to those of the control. Imery and Cequea (2001) explained that the chromosome doubling significantly increased cellular volume linked with plant width and thickness of the leaves. Likewise, the significant reduction of stomatal frequency in polyploid plants is considered a consequence of the stomata expansion caused by an increase in size in epidemic cells. It is generally considered that polyploids tend to have larger stomata compared to diploids (Munzbergova, 2017; Manzoor et al., 2019)
In spite of its natural accidental or random phenotypic changes, mutation breeding of succulents has been found to be a successful way to create new and attractive succulent cultivars that have significantly different morphological characteristics than their original forms. Jain (2006) and Schum (2003) emphasized that ideal ornamental mutant traits include new plant architecture, compact growth, and variegated leaves that are produced through cost-effective methods. These traits were evident in this study. The mutation-assisted breeding described here could contribute to the genetic improvement of plants and improve the socio-economy of horticulture and agronomy sectors of developing countries (Jain, 2006).
Thus, the use of colchicine in Echeveria species produced compact plants and, in some cases, chimera or alteration of leaf color. Further studies are recommended with other species or genera of succulent plants to consider the extent of their epicuticular wax and other distinctive features that may be affected after chemical mutagen treatment. In this study, we induced polyploid Echeveria species by colchicine treatment of leaf cuttings and obtained mutants with increased ornamental value on account of their unique leaf shape, color change, and compactness. It is expected that this treatment will be applied to other Echeveria cultivars or species and enable the production of new ornamental cultivars with novel morphological traits.