Introduction
Materials and Methods
Plant Material
Preparation of Explants
Culture Media and Culture Conditions
Callus and Shoot Induction
Callus and Shoot Proliferation
Adventitious Shoot Differentiation
Rooting of Shoots and Acclimatization
Statistical Analysis
Results and Discussion
Callus and Shoot Induction
Callus and Shoot Proliferation
Adventitious Shoot Differentiation
Rooting of Shoots and Acclimatization
Introduction
Haworthia is a succulent plant in the family Liliaceae (Kaul and Sabharwal, 1972; Beyl and Sharma, 1983), and Xanthorrhoeaceae and is endemic to southern Africa (Haworth, 1804). The family name was recently changed to Asphodelaceae (Chen et al., 2019; Kim et al., 2019). Economically, they are easy to culture and have a convenient size. However, compared with other succulent plants, they are more tolerant to modest lighting. They are commonly used as garden and container plants (Bayer, 1999). Haworthia plants are popular as ornamental plants because of the variation in their leave color. The leaves of Haworthia and Aloe are harder than those of Echeveria. The appearance of Haworthia plants is generally similar to miniature aloes, except for their flowers. The flowers are small, bell-shaped, and have green or brown lines. The inflorescence is a long thin stem with flowers on it, and for some species it may exceed 40 cm in height.
Haworthia succulents are unique compared to others and require different propagation methods. Haworthia can reproduce by seeds, leaf cuttings, crown divisions, forming pups, or by in vitro proliferation from leaves and inflorescences (Pilbeam, 1983). Propagation by root is an interesting method. The thick succulent roots need to be uncovered slightly, cut, and left to grow into new plants. For some species, new plants propagate from flower stem cuttings. The succulents are watered every 2 weeks in indirect sunlight. However, in vivo conditions are not suitable for mass propagation due to higher dormancy, small seeds, sophisticated work, and slower offset growing (Mycock et al., 1997). Micro-propagation is an efficient technique for breeding plants for ornamental and landscaping purposes (Preil et al., 1987; Rout et al., 2006; Kumari et al., 2016; Liu et al., 2017; Jang et al., 2019a; Jang et al., 2019b). With this technique, new varieties and virus-free plantlets can be obtained. Plantlets have been efficiently produced using Haworthia inflorescences(Majumdar, 1970a; Kaul and Sabharwal, 1972; Ogihara, 1981; Standifer and O'Rourke, 1984), ovary walls (Majumdar, 1970b), and leaves (Beyl and Sharma, 1983). In previous studies, 7 to 9 cm long young and immature inflorescences were used as a source of explants to generate shoots for monocotyledonous species (Richwine et al., 1995; Velcheva et al., 2005; Abul-Soad, 2012). According to Rogers (1993), somaclonal variation may occur in the callus of regenerated Haworthia plants. Direct shoot regeneration is less likely to lead to somaclonal variation and maintains genetic stability among propagules (Richwine et al., 1995). A previous study reported that, abscisic acid and kinetin have synergistic effects on the growth of callus from the inflorescence segments of Haworthia cultured in vitro(Kochhar, 1983; Loutfi et al., 1998).
In this study, 6-benzylaminopurine (BA) and naphthylacetic acid (NAA) were used for the regeneration of multiple shoots and roots. The objective of this study was to evaluate the response of different inflorescence sections of five cultivars and one species of Haworthia during tissue culture with different medium compositions. Our study could contribute to the development of an efficient mass propagation method using the inflorescences of Haworthia ‘Natalie’,‘Musin’, and ‘Tiffany × Fertenon B Com’ as explants.
Materials and Methods
Plant Material
The inflorescences of five commercial Haworthia cultivars and one species were collected for in vitro propagation from Dae Sun Farm, Goyang-si, Gyeonggi-do, South Korea in June 2018, which included Haworthia ‘Natalie’, ‘Musin’, ‘Tiffany × Fertenon B Com’, ‘Baeckbong’, ‘White Wolf’, and H.splendens.
Preparation of Explants
The inflorescences were used as explants and divided into three parts: U (upper), M (middle), and L (lower). The parts (U, M, and L) (10 cm long), were washed under running tap water. Then, they were shaken by hand with 1% NaOCl for 3 ‑ 5 min and washed five times with doubled- distilled water (Majumdar, 1970a; Richwine et al., 1995; Velcheva et al., 2005). On a clean bench, all explants were washed with 70% ethanol by hand for 60 s and washed five times with sterilized, distilled water. Subsequently, they were shaken by hand with 1% NaOCl + 2 drops Tween-20 for 10 min and washed five times with sterilized, distilled water. Finally, the explants were placed on a filter paper for drying. The two end portions of each part were discarded. Then, each part was cut into 1 to 1.5 cm long pieces using a sterilized surgical blade and inoculated on the medium.
Culture Media and Culture Conditions
Eight types of media were used for tissue culture (Table 1). For the preparation of media, Murashige and Skoog (1962) medium (MS medium) including vitamins (1 fold MS medium 4.4 g·L-1 and ½ fold MS medium 2.2 g·L-1) with/without growth regulators (kinetin and BA), sucrose (20.0 g·L-1), coconut water (20.0 ml·L-1), and Gelrite (2.0 g·L-1) was used (Table 1). In a previous study, the leaf explants of endangered Haworthia were cultured using kinetin/BA with NAA (Rogers, 1993; Giusti et al., 2002; Obsuwan et al., 2019). Instead of coconut milk, coconut water was used in all medium compositions both in 1 fold MS and in ½ fold MS media with/without growth regulators (Kaul and Sabharwal, 1972). Finally, the pH was adjusted to 5.8 followed by autoclaving at 121°C. However, 1 fold and ½ fold MS growth regulator free media were used for the inflorescences of Haworthia (Table 2). Of the inflorescences of the five cultivars and one species, two inflorescences were inoculated on 1 fold and ½ fold MS growth regulator treated media (Table 3). All cultures were incubated at 24 ± 1°C under a 16h photoperiod at a photosynthetic photon flux density of 40 µmol·m-2·s-1 using fluorescent lamps (Philips 35 W tubes).
Table 1.
Medium compositions used for the tissue culture of Haworthia inflorescences
| Medium | MS medium strength | Kinetin (mg·L-1) | BA (mg·L-1) | |
| 1 fold | ½ fold | |||
| H1 | 4.4 | - | 0 | 0 |
| H2 | 4.4 | - | 1 | 0.5 |
| H3 | 4.4 | - | 1 | 1 |
| H4 | 4.4 | - | 1 | 3 |
| H5 | - | 2.2 | 0 | 0 |
| H6 | - | 2.2 | 1 | 0.5 |
| H7 | - | 2.2 | 1 | 1 |
| H8 | - | 2.2 | 1 | 3 |
Table 2.
Effect of media on shoot and callus induction from inflorescences without growth regulators
yLC; lower cut end, IN; internode, UC; upper cut end, and E; upper, lower cut end and internode of the explant.
xNo shoot induction.
Table 3.
Effect of media on shoot and callus induction from inflorescences of H. splendens and Haworthia 'White Wolf' with kinetin and 6-benzylaminopurine (BA)
yLC; lower cut end, IN; internode, UC; upper cut end, and E; upper, lower cut end and internode of the explant.
Callus and Shoot Induction
Each treatment was repeated five times. After 20 days of inoculation, the shoot and callus induction percentage, days to shoot and callus formation, and callus location were recorded for three parts of the inflorescences. Then, the calli and shoots were used for proliferation. The poorly growing, brown-colored, abnormal calli and shoots were not used for proliferation but were preserved for future observation.
Callus and Shoot Proliferation
After 3 months of inoculation, the calli of all cultivars except Haworthia ‘Natalie’ were cut into 1 to 2 cm2 pieces and transferred to callus proliferation medium. The ½ fold MS medium supplemented with kinetin (1.0 mg·L-1), BA (0.5 mg·L-1), NAA (0.1 mg·L-1), sucrose (30 g·L-1), agar (7 g·L-1), was adjusted to pH 5.5. After proliferation, the calli were cut again into 2 cm2 pieces and cultured on shoot multiplication or growing medium (Liu et al., 2017). Shoots generated from the inflorescence explants of cultivars incubated with preliminary media were transferred to shoot multiplication or growing medium supplemented with ½ fold MS basal medium, BA (0.9 mg·L-1), sucrose (30 g·L-1), and plant agar (6 g·L-1) at pH 5.7 (Richwine et al., 1995). After obtaining the shoots from the calli of ‘Musin’ and ‘Tiffany × Fertenon B Com’ and the direct shoots of Haworthia ‘Natalie’, they were sub-cultured several times on shoot multiplication medium to increase the number of shoots.
Adventitious Shoot Differentiation
Shoots were obtained from shoot multiplication or growing medium and cultured on ½ fold MS medium containing only BA (0, 0.5, 0.9, and 1.4 mg·L-1) for adventitious shoot production. In the case of Haworthia ‘Natalie’, ‘Musin’, and ‘Tiffany × Fertenon B Com’, shoots were cultured on the shoot multiplication medium. The percentage of shoot induction, number of multiple shoots per plantlet, and length of shoots were recorded. Each treatment was repeated three times.
Rooting of Shoots and Acclimatization
Shoots with different sizes were inoculated on ½ fold MS medium supplemented with 0, 0.05, 0.08, or 0.1 mg·L-1 NAAfor shoot and root production. After inoculation for 4 weeks, the number of multiple shoots per plantlet, longest shoot length, percentage of root induction, number of roots per shoot, and longest root length were assessed. Each experiment was repeated three times. Rooted plantlets (2 ‑ 3 cm in length) were transferred to pots after 2 months of inoculation on the medium. Pots containing a mixture of vermiculite: perlite: soil (Veriflora peat moss) (1 : 1 : 2) were placed in a growth chamber (HB-301L-3, Hanbaek Scientific Co., Korea) at 25°C and 80% relative humidity under a 16h photoperiod.
Statistical Analysis
Callus induction was calculated according to the following formula; callus induction (%) = (no. of explants with callus / total no. of explants cultured) × 100. All data were analyzed using the statistical software SPSS (version 25; IBM). Significant differences among the treatments were determined by Duncan’s multiple range tests at p ≤ 0.05. The results are expressed as the mean ± SE of repeated experiments.
Results and Discussion
Callus and Shoot Induction
Callus and shoot induction occurred after 20 days of inoculation and was dependent on the different cultivars, inflorescence parts, and media. In other studies, the calli started to differentiate within 2 ‑ 3 weeks for Haworthia turgida. Haw. and Haworthia ‘Sansenjyu’, respectively (Liu et al., 2017; Chen et al., 2019). On H1 and H5 media, Haworthia ‘Natalie’ produced shoots and calli before 30 days after inoculation from three parts of the inflorescences (Table 2). Inflorescence explants developed yellowish-green calli with a compact texture for ‘Natalie’ (Fig. 1A). However, the calli died at 3months. According to Rogers (1993) and Phat (2017), the longest shoots were obtained from growth regulator free medium and medium containing the lowest kinetin concentration from leaf explants of H. comptoniana. ‘Musin’ and ‘Tiffany × Fertenon B Com’ produced the greatest percentage of shoots and calli on H5 medium from three parts of the inflorescences. For most treatments, shoot initiation occurred at the U part with bearded flowers under in vivo conditions. In this experiment, the H5 medium was better than the H1 medium both for shoot and callus induction, and the U part showed a greater response compared with other parts of the inflorescence without growth regulators. A previous study revealed that MS medium was more effective than ½ fold MS medium for callogenesis in rapeseed (Afshari et al., 2011). However, on H1 medium, no shoots were observed at the L part of the inflorescence for the two cultivars. ‘Musin’ developed dark-green colored shoots and fragile calli (Fig. 1B), and ‘Tiffany × Fertenon B Com’ developed vigorous yellow-green calli. However, in other studies, either 5.4 µM zeatin ribosome or 4 µM BA was used, and the shoots were obtained from both flower-bearing and non-flower-bearing axils (Richwine et al., 1995). In addition, without any plant growth regulators, the longest shoot was obtained from the two cultivars ‘Balumeise’ and ‘Vanilla Sky’ of hydrangea (Khaing et al., 2018). Moreover, the three other cultivars did not produce any shoots on H1 and H5 media (Table 2).
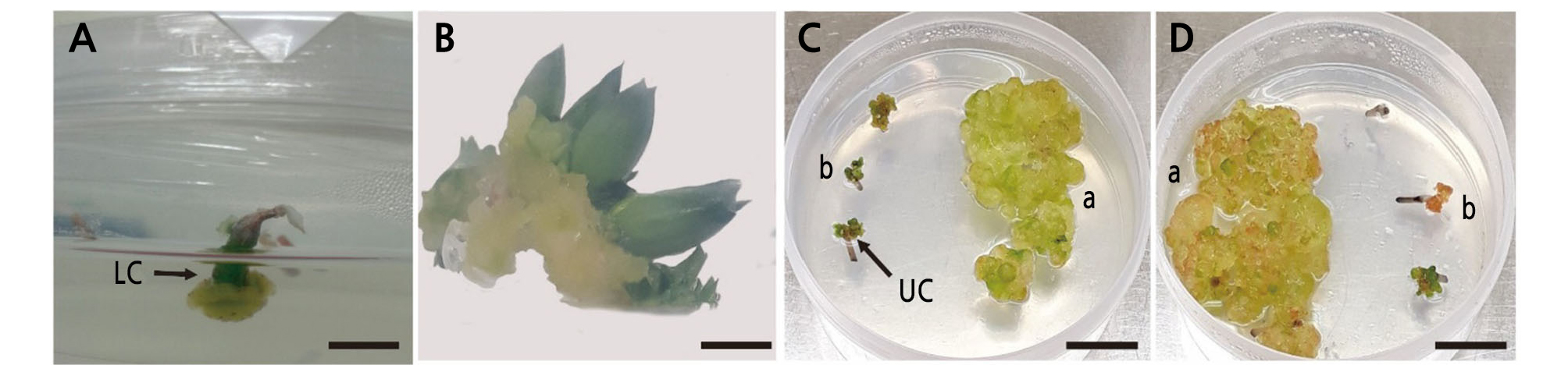
Fig. 1.
Morphogenetic responses of Haworthia explants. (A) Haworthia ‘Natalie’ on H1 medium produced calli in LC at 30 days after inoculation. (B) Haworthia ‘Musin’ on H5 medium produced shoots at the U part at 50 days after inoculation. Haworthia splendens (a) and Haworthia ‘White Wolf’ (b) on H6 and H7 media (C and D) at 3 months after inoculation formed calli at the L and U parts, respectively. Size bar: 0.5 cm.
The shoot and callus induction percentage was affected by kinetin and BA in H. splendens and ‘White Wolf’ (Table 3). Different media had a positive effect on the shoot and callus development of Haworthia cultivars and species in this study. The percentage of reactive inflorescence explants differed in various media for different explants. The two cultivars did not produce shoots on both media with or without growth regulators. Studies of chrysanthemum have demonstrated that more shoots were produced by direct plant regeneration using media containing 0.5 mg·L-1 BA compared with media containing 1.0 or 2.0 mg·L-1 BA. In this study, compared with Haworthia ‘White Wolf’, H. splendens produced more vigorous calli on growth regulator-treated media (H6, H7, and H8) (Fig. 1C and 1D). Medium combinations included ½ fold MS media supplemented with 1.0 mg·L-1 kinetin and 0.5, 1.0, or 3.0 mg·L-1 BA. However, callus formation was delayed using H6, H7, and H8 media compared with H2, H3, and H4 media, and larger calli were produced from H. splendens than from ‘White Wolf’.
Callus and Shoot Proliferation
After 3 months, the calli died, and shoots were transferred to shoot multiplication medium for Haworthia ‘Natalie’. After transferring the calli to callus proliferation medium, the calli of ‘Musin’ and H. splendens proliferated more vigorously compared with those of other cultivars after 2 weeks of inoculation. Then, the calli were transferred to shoot multiplication or growing medium. Shoots were regenerated from the calli of ‘Musin’ and ‘Tiffany × Fertenon B Com’. After 4 ‑ 6 weeks of inoculation, multiple shoots were developed from the calli (Fig. 2E and 2I). ‘Baeckbong’, H. splendens, and ‘White Wolf’ did not produce shoots on preliminary media. However, the initiated shoots from Haworthia ‘Natalie’, ‘Musin’, and ‘Tiffany × Fertenon B Com’ on preliminary media were transferred to shoot multiplication medium. The direct shoots of three cultivars on shoot multiplication medium died except for the shoots of the cultivar‘Natalie’ after 4 weeks of inoculation. Shoot multiplication medium was used for the multiplication and growth of shoots where the shoots were developed from the calli of ‘Musin’ and ‘Tiffany × Fertenon B Com’ and direct shoots of‘Natalie’. Therefore, multiple shoots were produced from the three cultivars.
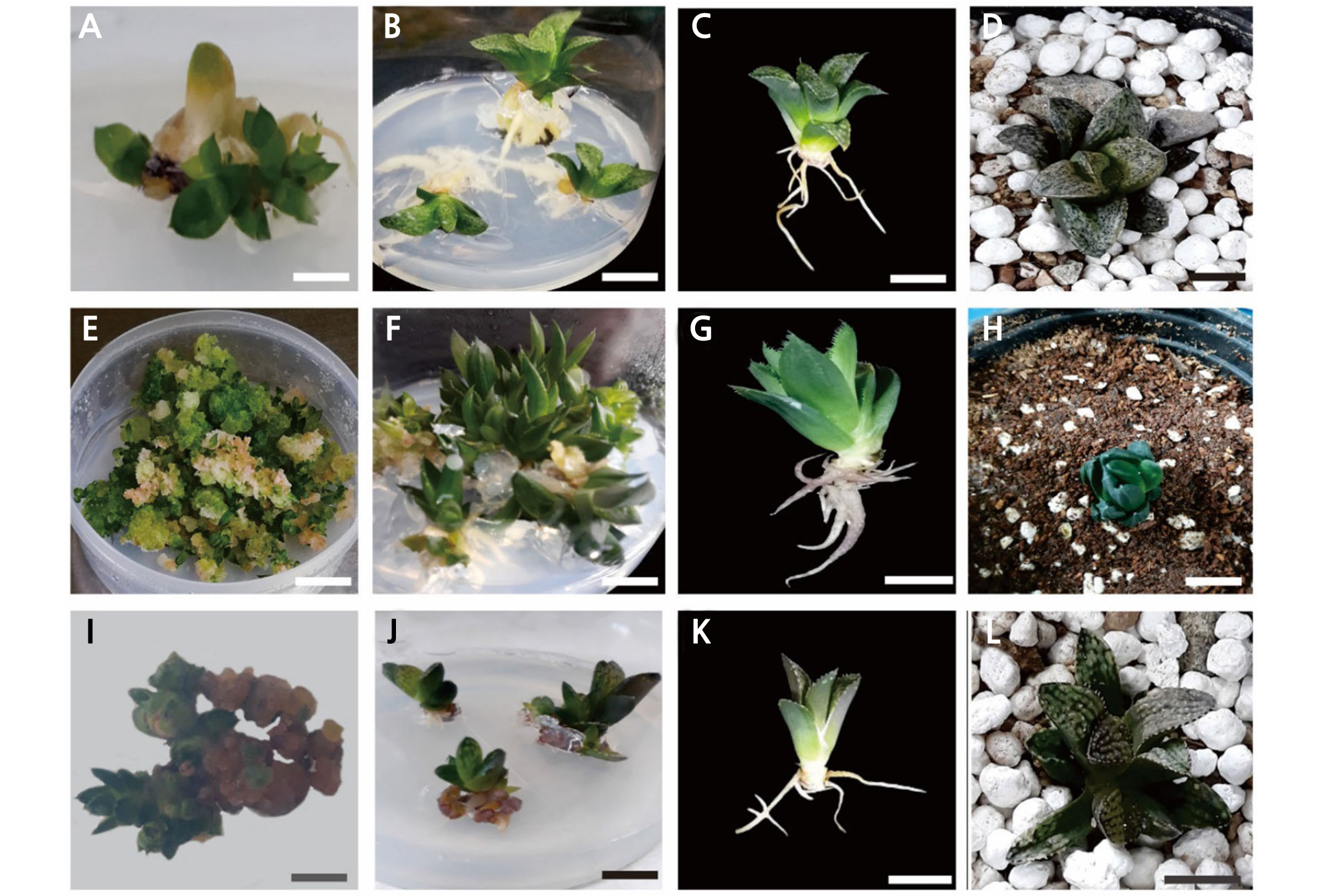
Fig. 2.
(A and B), Haworthia ‘Natalie’ developed multiple shoots and roots on ½ fold MS medium supplemented with 0.05 mg·L-1 NAA from micro-propagated plantlets. (C and D), before and after transfer to pots. (E) Haworthia ‘Musin’ developed multiple shoots from calli on shoot multiplication medium supplemented with 0.9 mg·L-1 BA. (F) ‘Musin’ developed multiple shoots on ½ fold MS medium supplemented with 0.08 mg·L-1 NAA from shoots (G and H), before and after transfer to pots. (I) Haworthia ‘Tiffany × Fertenon B Com’ developed shoots from calli on shoot multiplication medium (0.9 mg·L-1 BA) (J) ‘Tiffany × Fertenon B Com’ showed the lowest root induction (%) on ½ fold MS medium supplemented with 0.05 mg·L-1 NAA (K and L), before and after transfer to pots. Size bar: 2 cm.
Adventitious Shoot Differentiation
The shoots of Haworthia ‘Natalie’, ‘Musin’, and ‘Tiffany × Fertenon B Com’ were cultured on medium containing BA at different concentrations (0, 0.5, 0.9, and 1.4 mg·L-1). In the previous study, using 0.1 mg·L-1 BA resulted in more shoot regeneration and multiplication from stem t-TCL explants of H. cymbiformis than 0.5 or 1.0 mg·L-1 IAA (Iizumi and Amaki, 2011). The highest number of shoot multiplications (20.8 ± 0.29) was observed for ‘Tiffany × Fertenon B Com’ on medium containing 1.4 mg·L-1 BA (Table 4). In another study, MS medium supplemented with 1.0 ‑ 3.0 mg·L-1 BA alone was able to induce shoot production for Ficus benjamina vars. Natasja and Starlight (Rzepka-Plevnes and Kurek, 2000; Bae et al., 2005). According to Pérez-Molphe-Balch (2002), the transverse explants of the leaves of Carnegiea gigantea cultured on medium with 2 mg·L-1 BA showed the highest shoot efficiency compared with the efficiency of other species. Similar to our study, NAA or BA alone was less effective than combinations of BA and alone (Elias et al., 2015; Zhu et al., 2018). The response of Haworthia ‘Natalie’ was different from that of the other two cultivars. The direct shoots on BA- containing medium produced whitish, swollen basal shoots, which were not visible on NAA-containing medium for Haworthia ‘Natalie’. NAA was more effective at 1.0 mg·L-1 and the concentration of BA showed a correlation with shoot elongation media (Lu et al., 1990). Another study, reported that multiple shoots per explant were obtained, using MS medium containing 1.0 mg·L-1 BA and 0.5 mg·L-1 NAA for Ranunculus kazusensis (Park et al., 2017). Conversely, Haworthia ‘Musin’, and ‘Tiffany × Fertenon B Com’ did not produce swollen shoots and showed a better shoot induction percentage (96.3 ± 3.70% and 92.6 ± 3.70%, respectively).
Table 4.
Effect of 6-benzylaminopurine (BA) on adventitious shoot initiation in regenerated plantlets
Rooting of Shoots and Acclimatization
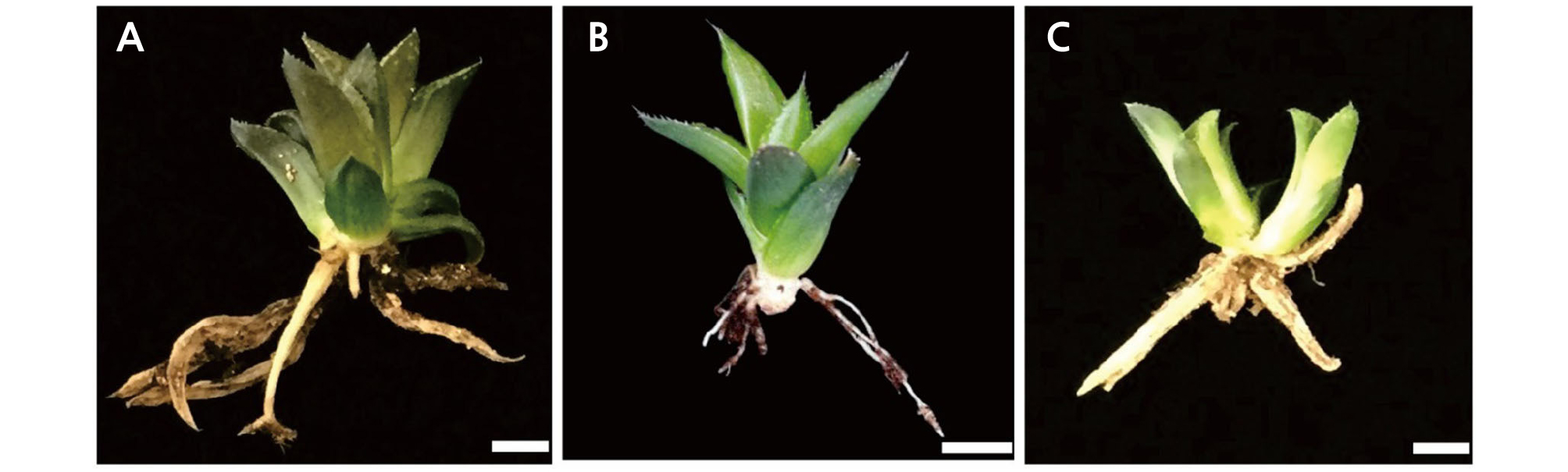
The shoots of the cultivar‘Natalie’ produced multiple shoots on medium containing 0.05 mg·L-1 NAA (Fig. 2A). However, 2 to 3 cm shoots did not produce multiple shoots but produced roots. The highest root induction percentage was observed for Haworthia ‘Natalie’ on ½ fold MS medium supplemented with 0.05 mg·L-1 NAA (Fig. 2B and 2C). The lowest root induction percentage was observed for ‘Tiffany × Fertenon B Com’ on ½ MS medium containing 0.05 mg·L-1 NAA (Fig. 2J). The highest number of multiple shoots per plantlet (13.5 ± 1.82) was achieved for ‘Musin’ on ½ fold MS medium containing 0.08 mg·L-1 NAA (Table 5 and Fig. 2F). In another study, compared with combinations with 0.5 mg·L-1 BA, 0.1 mg·L-1 NAA alone was able to produce the highest number (22.0) of shoots from calli (Kim et al., 2018). After acclimatization for 1 week, the rooted plantlets were washed with tap water and dried. Sixty plantlets were transferred to pots under greenhouse conditions. The pots contained a mixture of vermiculite: perlite: soil (1: 1: 2). The survival rates of Haworthia ‘Natalie’, ‘Musin’, and ‘Tiffany × Fertenon B Com’ were 100%, 93.3%, and 86.7%, respectively (Fig. 3A, 3B and 3C). Roots were regenerated from these plantlets under in vivo conditions after 4 weeks.
Table 5.
Effect of naphthylacetic acid (NAA) on adventitious shoot and root initiation in micro- propagated plantlets
It is useful to identify the optimal medium compositions for different Haworthia cultivars using inflorescence parts. In this study, only growth regulator-free MS medium and cytokinin were used. However, cytokinin and auxin combinations in the basal medium are important for shoot and callus formation. Organogenesis from Haworthia inflorescences may provide a new approach for the mass propagation of these cultivars. Several medium combinations could be used to obtain shoots and calli from different cultivars and species.