Introduction
Materials and Methods
Plant Materials and Preparation of Embryo Rescue
Culture Media and Sucrose and GA3 Concentration Supplementation
Genomic DNA Extraction and SSR Marker Analysis
Statistical Analysis
Results and Discussion
Fruit Set, Seed Formation, and Embryo Development
Embryo Germination and Rooting Response to Embryo Rescue Time and Culture Medium Type
Embryo Germination and Rooting Response to Sucrose and GA3 Concentration
Frequency of Zygotic Embryo Rescue through SSR Marker Analysis
Introduction
Korean citrus production predominantly comprises satuma mandarins (Citrus unshiu), which account for approximately 90% of total production (JSGP, 2016). The total production area of citrus has declined by approximately 28% from 26,234 ha in 2002 to 20,523 ha in 2015.The production of late maturing varieties reached 67,406 MT for 2,112 ha in 2015, an increase of 10% in the past 10 years. High yield production of satsuma mandarins combined with alternate year bearing and poor quality has repeatedly resulted in overproduction. This has led to price instability and cultivation decline. On the contrary, fruit imports in Korea have steadily increased due to the implementation of many Free Trade Agreements since 2003. Two major fruit imports were orange and banana, which have been popular with Korean consumers due to price competitiveness and good flavor. Therefore, it is urgent and essential for the Korean citrus industry to develop new, high-quality varieties with a high amount of bioactive components and a high tolerance to biotic and abiotic stresses to stay competitive in the market and satisfy not only consumer needs but also grower demand.
Historically, new varieties have mostly depended on the selection of nucellar seedlings (apomixis) and bud sports. The long juvenility from seed to flowering, complicated gene composition, polyembryony, gamete sterility, and self-incompatibility are significant barriers to developing new promising hybrid varieties in citrus breeding (Soost and Cameron, 1975). Therefore, it is necessary to develop methods to distinguish between zygotic and nucellar plants after sexual cross. Identification of sexual hybrids obtained from the cross using polyembryony maternal parents is difficult even though parental lines have convenient dominant traits (Golein et al., 2011). Although morphological (Cameron, 1979), infrared spectroscopy (Pieringer and Edwards, 1967), isozymatic (Anderson et al., 1991), chromatographic (Tatum et al., 1974), and molecular marker approaches such as restriction fragment length polymorphism (RFLP) (Carimi et al., 1998) and random amplified polymorphic DNA (RAPD) (Rodriguez et al., 2005; Yun et al., 2007; Mondal and Saha 2013) can be used to distinguish zygotic and nucellar seedlings, these methods are limited by low reproducibility or efficiency. Hence, advanced molecular markers such as simple sequence repeats (SSRs) (Oliveira et al., 2002; Tan et al., 2007; Yildiz et al., 2013) and inter-simple sequence repeat (ISSRs) (Golein et al., 2011) have been developed.
Embryo culture is one of the earliest forms of in vitro culture (Bridgen, 1994). Since the first attempt by Ohta and Furusato (1957), immature embryo rescue of Citrus has been useful in producing sexual hybrids through distinguishing zygotic and nucellar plants in many polyembryony types of Citrus (Carimi et al., 1998; Pérez-Tornero and Porras, 2008). In the Korean citrus breeding program, many polyembryony cultivars such as satuma, ponkan, and ‘Shiranuhi’ mandarins have also been widely utilized. These mandarins are known to have higher soluble solid contents, seedlessness, loose skin, or sweet taste (Matsumoto, 2001) and are attractive as breeding materials, except for polyembryony. Using RAPD analysis, Yun (2007) reported that the ‘Shiranuhi’ mandarin has a low frequency of sexual hybrids of 0.0 to 3.9%, which was lower than that of the satuma mandarin at 8.6 to 13.4%.
In this study, embryo rescue stage, culture medium, and sucrose and GA3 concentrations in the embryo culture were evaluated with the aim to improve the efficiency of obtaining sexual hybrids in ‘Shiranuhi’ mandarins with a polyembryony trait.
Materials and Methods
Plant Materials and Preparation of Embryo Rescue
Artificial pollination was performed at Hannong Bio Industry (HBI) Breeding Orchard in 2014 to 2016. The ‘Shiranuhi’ mandarin [(C. unshiu × C. sinensis) × C. reticulata] at full bloom stage was used as the maternal parent andthe ‘Sanguinelli’ blood orange(C. sinensis) was used as the paternal parent. Immature fruits of ‘Shiranuhi’ were harvested at 90, 105, 125, 145, and 180 days after pollination (DAP). After washing fruits carefully with tap water and blot-drying, fruits were immersed in 70% (v/v) ethanol for 30 min, rinsed three times with sterile distilled water, and dried on a clean bench. The fruits were cut open, and immature seeds were taken out by forceps. Immature embryos were separated carefully from the microphyll end of the seeds under a light microscope.
Culture Media and Sucrose and GA3 Concentration Supplementation
MS (Murashige and Skoog, 1962), MT (Murashige and Tucker, 1969), and B5 (Gamborg et al., 1968) media were used to investigate the effect of medium type on germination and rooting of embryos. Each medium contained 500 mg·L-1 malt extract (ME), 25 mg·L-1 adenine sulfate (ADS), 1 mg·L-1 GA3, 3% sucrose (w/v), and 0.8% phyto agar (w/v). The pH of the medium was adjusted to 5.8 before autoclaving (121°C for 15 min), and GA3 was added to the medium through filtering after autoclaving. The embryo culture was maintained at 25 ± 2°C under a 16-h-light/8-h-dark photoperiod. After 2 to 3 weeks, the germination and rooting rate of the embryos were measured. After that, the germinated embryos were transferred to MS medium supplemented with 1 mg·L-1 benzyl adenine (BA), 0.1 mg·L-1 napthalene acetic acid (NAA), 3% sucrose (w/v), and 0.8% phyto agar (w/v) at pH 5.8.
Different concentrations of GA3 and sucrose in the MS medium were also evaluated. Embryos at 90 DAP were placed on the MS media supplemented with sucrose (3, 5, 7, 9, and 12%) and GA3 (0, 0.5, 1.0, 2.5, and 5.0 mg·L-1). The survival, germination, and rooting rate of the embryos were measured 4 weeks after the embryo culture. After that, the germinated embryos were transferred to MS medium supplemented with 1 mg·L-1 BA, 0.1 mg·L-1 NAA, 3% sucrose (w/v), and 0.8% (w/v) phyto agar (w/v) at pH 5.8.
Genomic DNA Extraction and SSR Marker Analysis
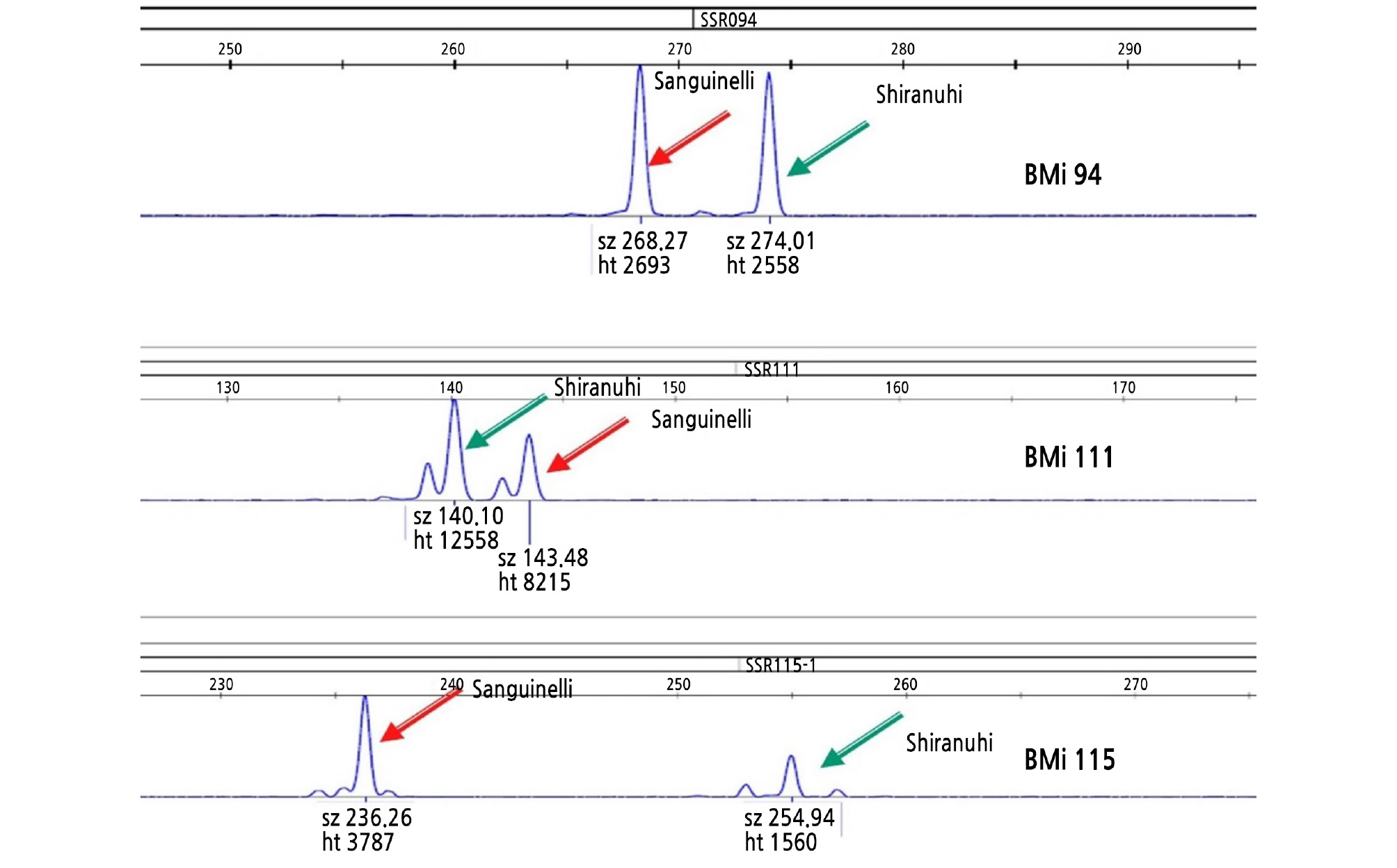
Genomic DNA was extracted from young leaves of the embryo-rescued plants using the Biomedic® gDNA Extraction Kit (Biomedic Co., Ltd., Korea). Genotyping was performed using the M13-tailed PCR method (Schuelke, 2000). Three SSR markers showing polymorphism between ‘Shiranuhi’ and ‘Sanguinelli’ were obtained from a previous report (Woo et al., 2019). The primers to amplify three SSR loci used in this study were as follows: BM-CiSSR94 forward (5’-TGTAAAACGACGGCCAGTGAATTGGGAGG ACGAACTGA-3’) and reverse (5’-CGAGCCCTAGACAGAGATGG-3’), BM-CiSSR 111 forward (5’-TGTAAAACGACGGCCAGTCCGATACAGCACAAAGCAAA-3’) and reverse (5’-TGGAAAGAGA GAAGCCAAGC-3’), and BM-CiSSR115 forward (5’-TGTAAAACGACGGCCAGTCGGTGTGTATTGGGTACACG-3’) and reverse (5’-TGGAAAGAGAGAAGCCAAGC-3’). PCR amplification was conducted using the ABI 2720 thermal cycler (Applied Biosystems) in a total volume of 10 mL containing 20 ng DNA, 5 mL 2x HSTM Taq mix (containing 0.3 unit/mL HS Taq DNA polymerase, 3.2 mM MgSO4, and 0.4 mM dNTPs) (Dongsheng Biotech, Guangzhou, China), each 0.2 mL of 10 pmol M13-tailed forward primer, 1 mL of 10 pmol reverse primer, and 1 mL of 10 pmol 6-FAM labeled M13 primer (5’-6-FAM-TGTAAAACGACGGCCAGT-3’). The conditions for PCR amplification were as follows: 5 min for initial denaturation at 94°C; 15 cycles of 30 s at 94°C, 30 s at 58°C, and 30 s at 72°C; followed by 20 cycles of 30 s at 94°C, 30 s at 53°C, and 30 s at 72°C; concluding with 1 cycle of 30 min at 72°C as a final extension reaction. The amplified fragments were separated by capillary electrophoresis on an ABI 3730 DNA analyzer (Applied Biosystems) using a 50-cm capillary with a DS-33 install standard as a matrix. Analysis of allele sizes was conducted using GeneMapper software (ver. 4.0; Applied Biosystems).
Statistical Analysis
The obtained data were analyzed using R console 3.2.3, and significant differences between means were assessed using Duncan’s multiple range tests at p ≤0.05.
Results and Discussion
Fruit Set, Seed Formation, and Embryo Development
Fruit sets of the ‘Shiranuhi’ mandarin cross-pollinated with the ‘Sanguinelli’ blood orange for 2014 ‑ 2016 were recorded with an average of 44.5% after a physiological drop, which had yearly variation depending on weather conditions (Table 1). Several studies reported that the fruit set of the cross-pollinated citrus might be affected by crossing parent and environmental conditions such as rootstock and climatic conditions (Soost and Cameron, 1975; Kedar and Gopal, 1977; Yelenosky, 1985; Sharma et al., 1999). The number of normal seeds per fruit ranged from 2.9 to 7.5 for 3 years with some variations. The number of normal seeds per matured fruit observed in this study was higher than those previously reported by Yun (2007), who obtained an average of 0.5 to 1.3 full seeds in ‘Shiranuhi, pollinated with ponkan mandarin and ‘Swingle’ citrumelo. This might have been caused by the difference in pollen genotype, but the exact reason requires further study.
Table 1. Fruit set and seed formation in the 'Shiranuhi' mandarin [(Citrus unshiu × C. sinensis) × C. reticulata] pollinated with 'Sanguinelli' blood orange (C. sinensis)
The total number of seeds ranged from 8.4 to 12.7 with some variation depending on the fruit development stage and DAP; the average number of seeds was the lowest, 8.4, at 180 DAP and the highest, 12.7, at 90 DAP (Table 2). The number of normal seeds decreased with increased DAP, whereas the number of abnormal seeds, the length of the seeds, and the number of embryos per seed were significantly increased as DAP increased. Furausato et al. (1957) showed that the correlation coefficient between the number of seeds contained in the fruit and the mean embryo number per seed fluctuated irregularly from year to year, from tree to tree, and from branch to branch, and no regularity could be observed. In sour orange, none of the embryos were observed in the immature seeds harvested at 65 ‑ 85 DAP, and the average number of dissected embryos per seed was significantly affected by developmental stage and genotype (Carimi et al., 1998). Therefore, it might be considered that the appropriate time for embryo rescue is different depending on citrus genotype.
Table 2. Seed and embryo development at different developmental stages of the 'Shiranuhi' mandarin [(C. unshiu × C. sinensis) × C. reticulata] pollinated with the 'Sanguinelli' blood orange (C. sinensis) cross
yMean separation within column by Duncan's multiple range test at p < 0.05.
The endosperm tissues were easily recognized by 125 DAP (Fig. 1). Peel coloration started at 180 DAP and, at that time, most seeds were in the form of a whole seed. This result indicated that there might be a correlation between coloration of the pericarp and full development of the seeds. The number of normal seeds in cross-pollinated fruits of the ‘Shiranuhi’ mandarin decreased as the fruits developed, whereas the number of abnormal seeds increased (Table 2). This phenomenon might be related to the natural seed degeneration process during fruit development. Button and Kochba (1977) indicated that polyembryony cultivars usually contain a zygotic embryo at the earlier stages of ovule and subsequent seed development. Nucellar embryos are most vigorous and so inhibit the full growth of the zygotic embryo and cause its degeneration before seed maturation.
Embryo Germination and Rooting Response to Embryo Rescue Time and Culture Medium Type
To examine the embryo germination response of the ‘Shiranuhi’ mandarin cross-pollinated with ‘Sanguinelli’ blood orange, embryos were excised from seeds at 90 ‑ 180 DAP and then were subjected to in vitro culture on MT, MS, and B5 media. The embryos developed into the cotyledon stage or shoots after 7 days of exposure to the medium (Fig. 3). The germination rate of the embryo increased as DAP increased and ranged from 22.0 to 76.1% with the lowest at 90 DAP (avg. 36.4%) and the highest at 145 DAP (avg. 74.9%) (Table 3). Rooting had not occurred in embryos obtained from 90 ‑ 105 DAP, whereas it was remarkably increased from 28.3% at 125 DAP to 64.8% at 180 DAP (Table 3). As a result, there was a significant difference in germination and rooting rate depending on embryo developmental stage and embryo rescue time with DAP, but there was no difference depending on culture medium type. These results indicated that embryo germination and rooting are strictly correlated with embryo development. This result was in accordance with a previous study (Carimi et al., 1998) that reported that there was a positive effect between embryo age and embryo germination, and the frequency of embryo germination and plant formation also increased with increasing age. Bridgen (1994) also reported that precocious germination (the germination of embryos before the completion of normal embryo development) causes the establishment of weak seedlings, which was similar to our result that showed low root activity at early embryo development stages.
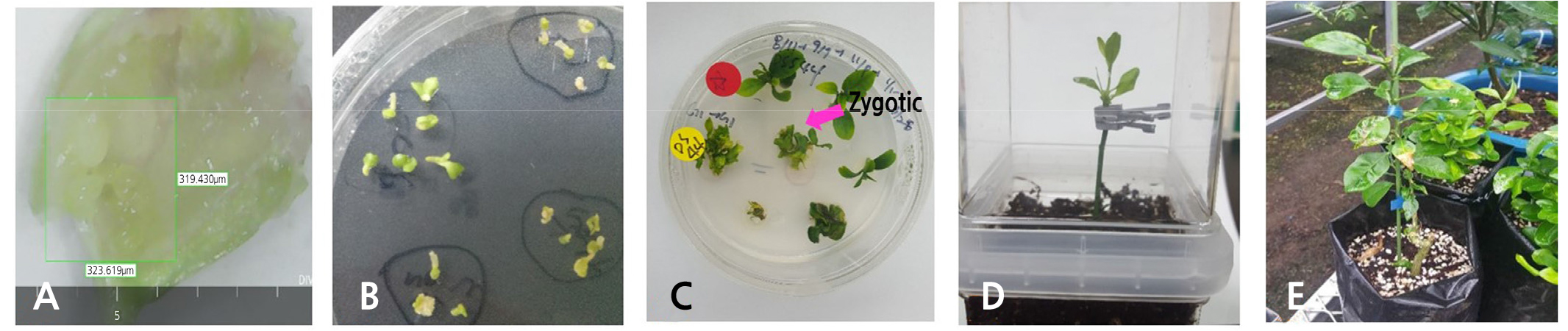
Fig. 3.
Overall process of embryo rescue from an initial stage of embryo culture to acclimatization stage by ex vitro grafting in the ‘Shiranuhi’ mandarin [(C. unshiu × C. sinensis) × C. reticula’] pollinated with the ‘Sanguinelli’ (C. sinensis) blood orange. (A) Embryo excised at 90 days after pollination (DAP). (B) Embryos cultured for 2 weeks in MS media containing 500 mg·L-1 malt extract (ME), 25 mg·L-1 adenine sulfate (ADS), 5 mg·L-1 gibberellin 3 (GA3), and 3% sucrose (w/v). (C) Embryos at the screening stage by the SSR marker analysis. (D) Ex vitro grafting of the zygotic origin plant on trifoliate orange rootstock. (E) Zygotic origin plant grown under greenhouse conditions.
Table 3. Effect of embryo developmental stage and medium type on embryo germination and rooting rate after 2‒3 weeks of embryo culture in the 'Shiranuhi' mandarin [(C. unshiu × C. sinensis) × C. reticulata] pollinated with the 'Sanguinelli' blood orange (C. sinensis)
NS,*,**,***Not significant or significant at 0.05, 0.01, or 0.001, respectively.
Embryo Germination and Rooting Response to Sucrose and GA3 Concentration
The essential role of GA on seed development can be inferred from transgenic and mutant studies in Arabidopsis, tomato, and pea, where depletion of bioactive GA from seed tissues caused abortion at early stages of development (Plackett and Wilson, 2016). Several studies have also reported that GA3 and sucrose were more useful for embryo rescue of Citrus (Carimi et al., 1998; Usman et al., 2002). In this study, it was investigated whether ‘Shiranuhi’ mandarin cross-pollinated with ‘Sanguinelli’ blood orange showed a similar response to sucrose and GA3 concentration (Table 4). The highest survival rate was 99.7%, and this was obtained from the combination of 12% sucrose and no supplement with GA3. The highest germination rate was 79.0%, and this was achieved with 5% sucrose and 5 mg·L-1 GA3. Survival and germination rate of embryos varied with combinations of sucrose and GA3 concentration, which showed a complicated response with no constant tendency. Rooting rate increased as sucrose concentration decreased while GA3 concentration was high (Table 4). The highest rooting rate 21.5% was obtained with the combination of 3% sucrose and 5 mg·L-1 GA3.
Table 4. Effect of sucrose and GA3 concentration on the survival, germination, and rooting rate of embryos obtained at 90 DAP from the 'Shiranuhi' mandarin [(C. unshiu × C. sinensis) × C. reticulata] pollinated with the 'Sanguinelli' blood orange (C. sinensis) after 4 weeks of embryo culture
NS,*,**,***Not significant or significant at p ≤0.05, 0.01, or 0.001, respectively.
Bridgen (1994) indicated that mature embryos usually grow well in medium with 2 ‑ 3% sucrose, whereas immature embryos grow better with a higher concentration of 8 ‑ 12%, which mimics the high osmotic potential within the young embryo sac. Usman et al. (2002) also reported that the germination rate of embryos in an interploidy hybridization of ‘Kinnow’ mandarin and ‘Succari’ orange was affected by GA3 concentration. GA3 plays various roles either promoting or inhibiting shoot and root formation depending on the species or conjunction with the auxin (Moshkov, 2008). Moshkov, Eshed et al. (1996) reported that pretreatment of bark of a 3-year-old stock oak tree (Quercus ithaburensis) with GA3 increased rooting over the control by 6- to 7-fold. Also, Ford et al. (2002) reported that the induction of adventitious rooting in cuttings of cherry (Prunus avium) was stimulated by GA3 pretreatment and that the number of roots per rooted cutting was increased up to 80% or more. Sagee et al. (1990) indicated a great enhancement of rooting in citrus by GA treatment in cuttings. Gibberellins are generally associated with juvenility, and application of gibberellins to the mature phase of several species might induce some juvenile characteristic (Hackett, 1985). Cuttings and tissue culture are vegetative propagation, and the response of immature rescued embryos to GA treatment might be similar to the response of cuttings. In this study, the embryo rescue response to sucrose and GA3 concentration was evaluated only at the young developmental stage from one genotype. Therefore, further study is required to determine how its response might be different with other genotypes and embryo developmental stages.
Frequency of Zygotic Embryo Rescue through SSR Marker Analysis
The zygotic individuals from the sexual cross between the ‘Shiranuhi’ mandarin and the ‘Sanguinelli’ blood orange were selected with SSR markers developed previously (Woo et al., 2019). Fig. 2 shows a representative SSR analysis, and the results are represented in Table 5. The zygotic embryo percentage per seed ranged from 12.1% at 90 DAP to 1.0% at 145 DAP and 4.1% at 180 DAP with a decrease as embryos developed further. This result showed that zygotic individuals could be selected at a higher rate from embryo rescue at the early stage compared to that at the late stage.
Table 5. Frequency of zygotic origin embryo rescued at different embryo developmental stages and identified by SSR marker analysis in the 'Shiranuhi' mandarin [(C. unshiu × C. sinensis) × C. reticulata] pollinated with the 'Sanguinelli' (C. sinensis) blood orange
yThe ratio of zygotic origin was calculated as the total number of SSR genotyping detected shoot per total number of seeds or embryos evaluated × 100.
Several studies have reported various zygotic frequencies in other citrus genotypes depending on the maternal and pollen genotypes, which ranged from 0 ‑ 87% (Frost and Soost, 1967; Hwang, 1991; Bastianel et al., 1998; García et al., 1999; Ruiz et al., 2000; Yun et al., 2007; Yildiz et al., 2013; Jin et al., 2015). Andrade-Rodríguez (2004) reported that the zygotic embryos were located near the micropylar end and were the smallest mature seeds of C. volkameriana. In contrast, Yun (2007) showed that positioning patterns of hybrids of mature ‘Miyagawa Wase’ and ‘Okitsu Wase’ are different depending on the cultivar. In seeds of sour orange collected at 125 ‑ 220 DAP, a zygotic embryo was mixed with many nucellar embryos, and they could not be distinguished from each other on the bases of size and position in the seed (Carimi et al., 1998). These results showed that there is a limit to the morphological selection method based on size and location of embryos cultured from mature citrus seeds.
Rangan et al. (1969) could successfully select hybrid plants from the polyembryonic sour orange C. aurantium. Sexual hybrid plants were obtained from the embryos at the heart-shaped stage of immature seeds at 100 ‑ 120 days after anthesis. They also reported that varieties containing few embryos showed the formation of more seeds with monoploidy and more healthy development of zygotic embryos, further increasing the zygotic ratio. Carimi et al. (1998) also mentioned that the zygotic embryo rescue appeared to be dependent on the number of nucellar embryos. Lower numbers of nucellar embryos per seed usually results in a larger dimension and greater probability of zygotic embryo survival. Our results also showed that the number of embryos from the ‘Shiranuhi’ mandarin cross-pollinated with the‘Sanguinelli’ blood orange was remarkably increased as DAP increased, and the higher rate of the zygotic embryo was obtained from immature embryo rescue at the earlier stage.
In conclusion, immature embryo rescue could be a preferred method for early selection of citrus hybrids. The reason for its superiority is that citrus plants take several years to bear fruit, which makes it difficult to conduct early characterization and requires a large-scale experimental field, as well as considerable time and effort to manage them. The goal of citrus breeding in Korea is to improve the sugar content, eradicate the seed, and shorten the time to maturity, in addition to increasing the aroma and resistance to pests and disease. The C. unshiu fruits have valuable traits such as seedlessness, early maturity, and a rind that is easy to peel. Therefore, it would be interesting to introduce our method to C. unshiu and determine if it can improve selection of zygotic plants.