Introduction
Materials and Methods
Fruit Material and Experimental Design
Gaseous ClO2 Fumigation Treatment
Fruit Decay and Cracking Incidence
Analysis of Microbial Activity
Determination of Fruit Firmness and Soluble Solids Content (SSC)
Weight Loss
Statistical Analysis
Results and Discussion
Effect of ClO2 on Jujube Fruit Quality Attributes during Cold Storage
Effect of ClO2 Treatment on Storage Disorder of Jujube Fruit
Effect of ClO2 Treatment on Microbial Growth
Correlation of Fruit Quality Attributes and Microbial Growth
Conclusion
Introduction
Jujube (Ziziphus jujuba Mill.), belonging to the family Rhamnaceae, is distributed in subtropical and tropical regions of Asia, Russia, northern Africa, southern Europe, and the Middle East (Hernández et al., 2014). In Korea, dried jujube fruit have long been used in traditional medicine since it is enriched in vitamins, minerals, flavonoids, and other organic compounds (Guo et al., 2015). It has been shown to be effective in stimulating the immune system and in treating insomnia, cancer, and inflammation (Li et al., 2011; Plastina et al., 2012; Yu et al., 2012). Recently, the popularity of fresh jujube fruit has increased owing to its sweet flavor and crisp texture. However, fresh jujube fruit is susceptible to low temperature which is responsible for chilling injury and also easily get postharvest disease caused by several fungal and bacterial pathogens, such as Alternaria alternata, Botrytis cinerea, Penicillium expansum,and Xanthomonas arboricola (Wang et al., 2009; Myung et al., 2010; Liu et al., 2018). As these pathogens cause considerable economic loss, when infecting the jujube fruit during cold storage, synthetic chemical fungicides are usually used for pathogen control. However, public concerns have been raised regarding the potential impact of fungicides on human health and the environment (Cohen, 2008).
Chlorine dioxide (ClO2) is a strong oxidizing and sanitizing agent with an oxidation capacity approximately 2.5-times higher than chlorine and is thus widely used to control microbiological growth in several industries (Wu and Rioux, 2010; Lee et al. 2012). Toxicity of gaseous ClO2 is reported to be minimal at concentrations used for decontamination (Gordon and Rosenblatt, 2005). ClO2 is particularly effective at controlling Salmonella, Escherichia coli O157:H7, Listeria monocytogenes, yeasts, and molds growing on raw food materials (Behrsing et al., 2000; Park and Kang, 2018). Gaseous ClO2 is considered to be effective at reaching and inactivating pathogenic cells owing to its high diffusivity and penetrability (Gomez-Lopez, 2012; Sun et al., 2017). Gaseous ClO2 has been applied to various fruits for pathogen control and retention of freshness during storage, including apples, green peppers, strawberries, and tomatoes, at different concentrations, relative humidity, and time combinations (Han et al., 2000; Du et al., 2003; Mahmoud et al., 2007; Trinetta et al., 2010). Several studies have demonstrated clear reductions in the total population of bacteria, yeast, and mold (Trinetta et al., 2013; Park and Kang, 2015; Kim and Hwang, 2016). Vandekinderen et al. (2009) reported that the mean reductions of gram-negative bacteria, such as E. coli and S. enterica Typhimurium, showed 3.5 log CFU/cm2, while gram-positive bacteria, such as L. monocytogenes and Lactobacillus sakei and yeast/mold were 2.6 and 0.9 log CFU/cm2, respectively. Generally, gaseous ClO2 is more effective against gram-negative bacteria than gram-positive bacteria, with an intermediate effect against mold and yeast. Gaseous ClO2 treatment substantially reduced Colletotrichum acutatum and E. coli populations after 10 d of cold storage in blueberries (Sun et al., 2014). In winter jujube, ClO2-treated fruit showed higher vitamin C content and lower soluble pectin content, which has a positive effect on fruit quality (Zhang and Zhang, 2006). Fu et al. (2019) found that ClO2 controls B. cinerea in jujube fruit, the causal agent of gray mold in various fruit, by disrupting the cell membrane of the fungal pathogen. However, there has been a lack of research on the effectiveness of gaseous ClO2 treatment in preventing microbiological spoilage of postharvest jujube fruit during cold storage. In this study, we investigated the effectiveness of different ClO2 gas concentrations in controlling decay and microbial growth on jujube fruit during 6 weeks of cold storage.
Materials and Methods
Fruit Material and Experimental Design
‘Bokjo’ jujube (Ziziphus jujuba Mill.) fruit were hand-harvested on October 10, 2018, at maturity (more than 70% colored fruit) from commercial orchards in Boeun, Republic of Korea. The fruit were selected for uniformity of size, color, ripeness, and lack of physical injury (fruit weight: 14.1 ± 1.5 g; soluble solids content: 25.3 ± 2.9% Brix and firmness: 29.4 ± 5.1 N). The fruit were immediately transported to the laboratory within 3 h and randomly divided into four sets of 5-kg samples (35 fruits per sample) for each concentration treatment. The fruits were carefully put into a plastic box and then exposed to gaseous ClO2. The fruit quality assays were examined at 0, 1, 2, 4, and 6 weeks of cold storage.
Gaseous ClO2 Fumigation Treatment
The four sets of fruit were fumigated with 0 (as a control), 10, 30, or 50 mg·L-1 ClO2 gas for 30 min under a relative humidity of 80% to suppress decay and microbial growth (Kang et al., 2015; Kim and Song, 2017). A ClO2 generator (CA-300, Purgofarm Inc., Hwaseong, Korea) was used employing the electrochemical method reported by Gates (1998), in which highly pure ClO2 gas produced by aqueous sodium chlorite was passed through a multi-porous membrane electrode assembly. After these electrochemical reactions, the ClO2 gas was flowed through a vent into a collection chamber for the fumigation treatment. After fumigation at harvest, each treated set was sealed in plastic containers (184 ×129 × 70 mm, Easepack, Namyangju, Republic of Korea) and then stored at 2 ± 1°C under a relative humidity of 80%, which is the condition generally used in commercial jujube orchards, for up to 6 weeks.
Fruit Decay and Cracking Incidence
The effects of fumigation on the incidences of fruit decay and peel cracking were evaluated using the number of infected and/or moldy and cracked fruit. These counts were taken at the beginning of the experiment and after 1, 2, 4, and 6 weeks of cold storage. Seventy fruit were used for the measurement of the decay incidence for each treatment.
Analysis of Microbial Activity
The microbial activity on the jujube fruit surface was quantified at 0, 1, 2, 4, and 6 weeks after the application of fumigation treatment. For the assessment of total microbial growth, 3 g of jujube pericarp from 1 g per fruit was mixed with 27 mL of distilled water, and this mixture was used to prepare a 3-fold serial dilution. Each diluent (1 mL) was inoculated in triplicate on 3M Petrifilm Aerobic Count Plates and 3M Petrifilm Yeast and Mold Count Plates (3M Co., Saint Paul, MI, USA). The aerobic bacteria were then incubated at 37°C for 48 h, and the yeast and mold were incubated at 25°C for 5 d. After incubation, total microorganisms were counted visually, based on the numbers of red-, blue-, and black-colored colonies for the total bacterial, yeast, and mold count, respectively. Each microbial determination was performed in triplicate, and the results were expressed in log colony-forming units (CFU) per gram.
Determination of Fruit Firmness and Soluble Solids Content (SSC)
The firmness and SSC of the fruit were determined at 0, 1, 2, 4, and 6 weeks of cold storage. Flesh firmness was measured using a penetration test with a 5-mm-diameter probe to a depth of 5 mm using a rheometer (CR-3000EX-S; Sun Scientific Co., Tokyo, Japan), defined as the maximum penetration force (N). Fruit juice was extracted by homogenizing one fruit in a juicer, resulting in individual SSC values. The SSC (%) was then measured using a digital refractometer (RA-510; Kyoto Electronics MFG Co., Tokyo, Japan).
Weight Loss
To measure weight loss, each jujube fruit was weighed on a digital scale (AUX220; Shimadzu Co., Japan) at the beginning of the experiment and after storage. Results are expressed as the percentage loss of the initial weight.
Statistical Analysis
The experiments were set up using a completely randomized design with three replicates (10 fruit were used for each replicate) and data expressed as mean ± standard error. Data were analyzed by one-way analysis of variance (ANOVA) using Statistical Package for the Social Sciences (SPSS) software version 18.0 (SPSS, Inc., Chicago, IL, USA). The MetaboAnalyst (v 4.0) online program (https://www.metaboanalyst.ca/) was used to calculate the correlation coefficients among parameters and for data visualization (Chong et al., 2019). Regression analysis was conducted using Sigma Plot 12.0 software (Systat Software, CA, USA). A difference was considered to be statistically significant at P < 0.05.
Results and Discussion
Effect of ClO2 on Jujube Fruit Quality Attributes during Cold Storage
Fumigation with ClO2 gas is known to be effective in maintaining fruit quality by suppressing microbial growth and reducing decay in various fruits and vegetables (Han et al., 2000; Lee et al., 2004; Park and Jeong, 2015). Therefore, this study aimed to evaluate the effectiveness of ClO2 gas fumigation treatment in controlling fruit decay during cold storage in jujube fruit.
As shown in Fig. 1, there was an obvious increase in quality with higher concentrations of ClO2 treatment, especially at 30 and 50 mg·L-1. Fruit firmness is one of the most representative indicator to monitoring the quality of fruit according to ripening and/or softening (Valero et al., 2007). There was no significant difference in flesh firmness of fumigated fruit based on the concentrations of gaseous ClO2 applied (Fig. 2A). However, there was a significant difference in fruit firmness depending on storage duration; the values decreased from 30.0 N to approximately 24.0 N throughout the 6 weeks of cold storage. Yan et al. (2012) also showed that firmness of jujube fruit gradually decreased as storage time increased. The SSC in jujube fruit showed a consistent value (range 25 ‑ 28%), regardless of the ClO2 concentrations applied or storage duration (Fig. 2B). The weight loss of fresh jujube fruit treated with different concentrations of ClO2 gas fumigation significantly increased with prolonged storage, while there was no significant difference according to ClO2 concentration (Fig. 2C). Though the treatment of ClO2 is known to inhibit weight loss of fruit and vegetables in general, Sun et al. (2014) reported that gaseous ClO2 did not affect weight loss in fresh blueberries, which is a similar result as ours.
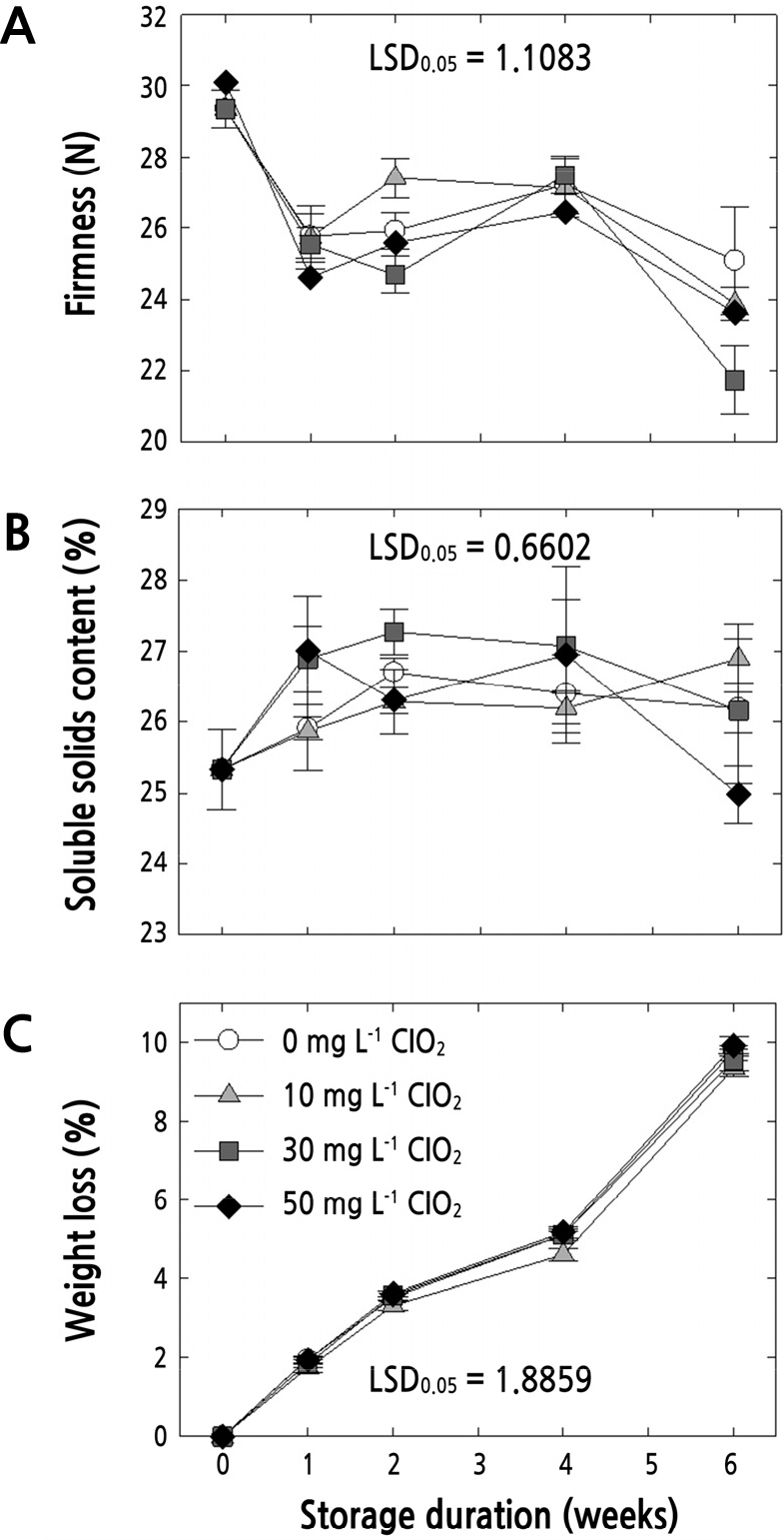
Fig. 2
Fruit firmness (A, n=30), soluble solids content (B, n=30), and weight loss (C, n=5) in fresh ‘Bokjo’ jujube fruit treated with 0, 10, 30, or 50 mg·L-1 gaseous chlorine dioxide (ClO2) at harvest, followed by storage at 2 ± 1°C for up to 6 weeks. Vertical bars represent the standard errors of replicates.
Effect of ClO2 Treatment on Storage Disorder of Jujube Fruit
The decay incidence of jujube during the storage period after fumigation treatment is shown in Fig. 3A. The control group showed rapid decay, with the incidence increasing from 2.8% at 1 week of cold storage to 18.3% at 4 weeks, whereas decay incidence in the groups treated with 30 mg·L-1 and 50 mg·L-1 was below 7% until 4 weeks, representing a significant reduction compared to the control group. At the end of storage duration, decay incidence of the 50 mg·L-1 group was controlled at <20%, whereas more than 40% of the fruit in the control group showed symptoms of decay. Thus, 50 mg·L-1 was the most effective concentration to prevent the development of fruit decay and decay incidence decreased in parallel with the increase in gaseous ClO2 concentration (Fig. 4A). Similarly, Du et al. (2007) showed that the efficacy of ClO2 gas on inhibiting the decay of green bell pepper was elevated with the increase in ClO2 concentration; after 40 d of ClO2 gas treatment, the rates of decay in bell pepper stored at 10 ± 0.5°C were 50% lower than those of the control. Also, the natural incidence of decay in fig fruit, mostly due to gray mold rot, was shown to be significantly reduced after ClO2 fogging (Karabulut et al., 2009). Fruit cracking was occasionally observed during cold storage, which was reported as one of the main factors contributing to provoking fruit decay and then deteriorating fruit quality and storability (Hua et al., 2015). We first noted fruit cracking at 1 week of cold storage, with an incidence of about 2% in all the treated groups; subsequently, the cracking rates increased to approximately 15% by the end of storage duration (Fig. 3B). However, there was no influence of ClO2 concentration on the incidence of fruit cracking.

Fig. 3
Decay incidence (A, n=30), cracking (B, n=30), total bacterial growth (C, n=3), and total yeast and mold growth (D, n=3) in fresh ‘Bokjo’ jujube fruit treated with 0, 10, 30, or 50 mg·L-1 gaseous chlorine dioxide (ClO2) at harvest, followed by storage at 2 ± 1°C for up to 6 weeks. Vertical bars represent the standard errors of replicates.
Effect of ClO2 Treatment on Microbial Growth
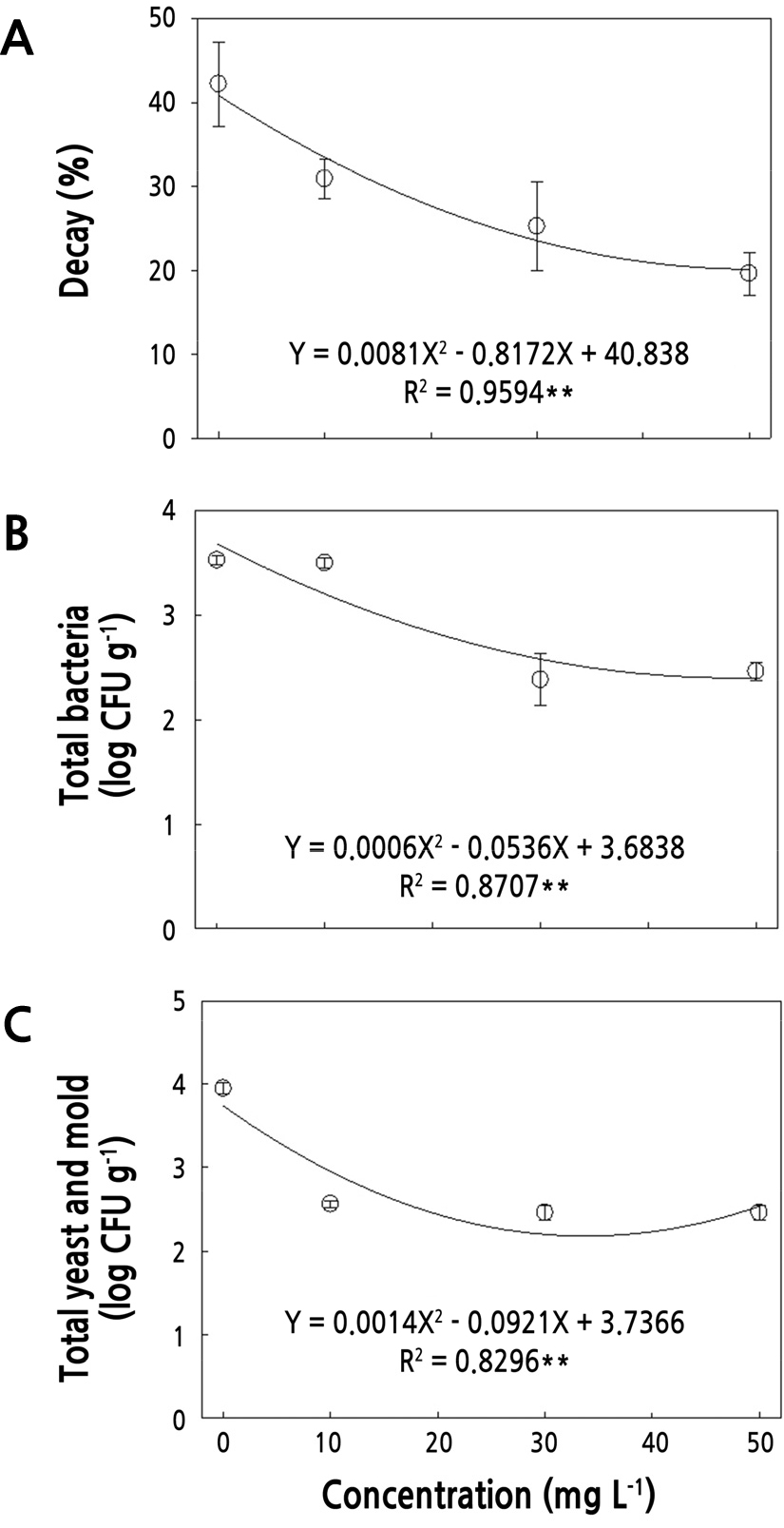
The total bacteria count in the control group increased from 1.3 to 3.5 log CFU·g-1 during cold storage; however, no bacterial growth was observed in the group treated with 50 mg·L-1 of gaseous ClO2 until 14 d of cold storage (Fig. 3C). After 6 weeks, the bacterial growth in the groups treated with 30 mg·L-1 and 50 mg·L-1 ClO2 was 30% lower than that of the control and the 10 mg·L-1 ClO2 groups, representing a significant difference (p < 0.01); the 30 mg·L-1 and 50 mg·L-1 ClO2 treatment groups had an average bacterial count of only 2.4 log CFU·g-1 in contrast to around 3.5 CFU·g-1 in the control and 10 mg·L-1 groups. The gaseous ClO2 fumigation treatment, especially at 50 mg·L-1, effectively prevented the growth of bacterial colonies on jujube fruit until 2 weeks. Similarly, ClO2 fumigation for 30 min at 20 ppmv was reported to be sufficient to inhibit E. coli, S.typhimurium, and Listeria monocytogenes on tomatoes and spinach leaves (Park and Kang, 2018). Sun et al. (2017) also found that 5 mg·L-1 of ClO2 completely suppressed the growth of E. coli and Penicillium digitatum in a chamber experiment. ClO2 is considered to decrease bacterial colonies by damaging and destabilizing the inner cellular membranes, thereby contributing to altering the cellular permeability of microorganisms, causing cell metabolism disruption (Young and Setlow, 2003; Vandekinderen et al., 2009). The total bacteria count decreased in a quadratic manner with the increase in ClO2 concentration (Fig. 4B). Similar to our result, the log reduction of S. enterica Typhimurium on grape fruit and L. monocytogenes on strawberry increased as the ClO2 gas concentration increased (Mahmoud et al., 2007; Netramai et al., 2016).
Fumigation with gaseous ClO2 significantly reduced the yeast and mold count (p < 0.001), compared with those of the untreated control. Although there was no significant difference in efficacy based on ClO2 concentrations, fumigation with more than 10 mg·L-1 of gaseous ClO2 was clearly effective in suppressing yeast and mold growth in jujube fruit during cold storage (Fig. 3D). ClO2 gas fumigation treatments also previously showed strong antifungal activity against Alternaria alternata on tomatoes for 3 min at 10 mg·L-1 (Trinetta et al., 2013). Similarly, Mahmoud et al. (2007) reported that treatment with 5 mg·L-1 ClO2 for 10 min significantly reduced the initial populations of yeast and mold on strawberry fruit from 4.8 log CFU·g-1 to an undetectable value. Fu et al. (2019) demonstrated that as the ClO2 concentration increased, the disease diameter caused by Botrytis cinerea was significantly reduced, indicating the effectiveness of ClO2 fumigation against B. cinerea and a positive correlation with respective concentrations. The mechanism of antifungal activity has been considered to be involved in the direct inhibition of ClO2 on mycelial growth, spore germination, and germ tube elongation of B. cinerea and Penicillium expansum, along with the induction of cellular damage to integrity of the plasma membrane, causing leakage of fungal cellular contents (Zhang et al., 2018; Fu et al., 2019). Total yeast and mold counts were lower even at 10 mg·L-1 ClO2 treatment; gaseous chlorine dioxide treatment was highly effective in controlling total yeast and mold counts even at low concentration (Fig. 4C).
Correlation of Fruit Quality Attributes and Microbial Growth
Pearson’s correlation coefficient heatmap matrix among fruit quality attributes was constructed to evaluate the overall responses to the four concentrations of ClO2 gas (Fig. 5). Firmness was negatively correlated with fruit decay, peel cracking, and weight loss (p < 0.0001). Wang et al. (2019) found similar results as fruit firmness decreased during cold storage, while aerobic plate count (APC) and yeast and mold counts increased, indicating a negative correlation between firmness, APC, and yeast and mold count. In contrast, yeast, mold, and bacterial growth; weight loss; peel cracking; and fruit decay were highly positively correlated with each other. Kong et al. (2013) also reported that the injury from side cracking and ostiole-end splitting in fig fruit accelerated development of decay and loss of marketability. The damage from fruit cracking and splitting that occurs over the storage duration usually is a starting point for infection and water loss.

Fig. 5
Pearson’s correlation coefficient (r) matrix among fruit quality attributes of jujube fruit fumigated with 0, 10, 30, or 50 mg·L-1 gaseous chlorine dioxide (ClO2) at harvest followed by storage at 2 ± 1°C for up to 6 weeks. Red and blue indicate positive and negative correlation coefficients between variables, respectively. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. SSC stands for soluble solids content.
Conclusion
This study demonstrated that postharvest treatment with 30 ‑ 50 mg·L-1 gaseous ClO2 fumigation for 30 min can significantly control the natural infection and inactivation of microorganisms in cold-stored jujube fruit, thereby preventing fruit decay and ensuring quality. Overall, ClO2 gas fumigation treatment could effectively extend the storability of jujube fruit by inhibiting the incidence of fruit decay and bacterial/fungal growth as well.