Introduction
Materials and Methods
Plant Material and Growing Conditions
Chemical Treatments and Evaluation of Sexual Expression
Results
Effects of Ethrel, AVG, and AgNO3 on the Floral Development of Different Melon Genotypes
Effects of Ethrel on Sex Expression in Different Melon Genotypes
Discussion
Introduction
Melon (Cucumis melo L.) belongs to the genus Cucumis, family Cucurbitaceae. It is an economically important vegetable crop cultivated worldwide and a model species for studying sex determination. Understanding floral development and sex expression is necessary for melon and hybrid seed production. Melon exhibits diverse sexual phenotypes, including various combinations of three types of flowers: bisexual (hermaphrodite), female (pistillate), and male (staminate). Commercial cultivars of melon belong mostly to the andromonoecious type and partly to the monoecious and hermaphroditic types. Other sex types include gynomonoecious, trimonoecious, gynoecious, and androecious (not naturally available). Therefore, the production of most commercial melons requires laborious hand emasculation to produce hybrid seeds. It is necessary to develop monoecious/gynoecious sex types in elite germplasm to obviate self-pollination; however, breeding monoecious or stable gynoecious melon inbred lines with acceptable fruit quality is not easy. To overcome the problems related to mechanical emasculation, determining an exogenous hormone application that will induce female flower production in melon without causing any adverse effects on plant growth and environment is highly desirable.
Sex expression in melon is a complex process influenced by genetic, hormonal, and environmental factors, as well as their interactions. Recently, the involvement of ethylene in sex determination in melon was genetically demonstrated by the isolation and characterization of the main sex determination genes: the andromonoecious gene CmACS7 (locus m), the gynoecious gene CmWIP1 (locus g), and the androecious gene CmACS11 (locus a) (Boualem et al., 2008; Boualem et al., 2009; Martin et al., 2009; Boualem et al., 2015; Boualem et al., 2016). Furthermore, allele gy interacts with m and g to produce stable gynoecious plants in melon (Kenigsbuch and Cohen, 1990). Different allelic combinations of these genes result in several distinct sex phenotypes in melon: hermaphroditic (_ _ggmm), monoecious (A_G_M_), and andromonoecious (A_G_mm). However, it is not known how male and female flowers coexist on the same plant in monoecious species and how purely male plants can emerge from monoecious or hermaphrodite populations (Boualem et al., 2015).
In addition to genetic control, sex expression in melon can be modified by external factors, such as mineral nutrition, temperature, water restriction, light intensity, photoperiod, mechanical trauma, and application of growth regulators (Freeman et al., 1980; Girek et al., 2013). There are various studies on the involvement of growth regulators and phytohormones in melon sex determination from the past several decades (Noguera et al., 2005; Papadopoulou et al., 2005; Yamasaki et al., 2005; Little et al., 2007; Boualem et al., 2008; Girek et al., 2013). Exogenous application of ethylene-related chemicals has led to increased femaleness (Rudich et al., 1969; Karchi, 1970). Inhibition of ethylene biosynthesis with aminoethoxyvinylglycine (AVG) or perception with silver ions leads to increased maleness after application (Owens et al., 1980; Nijs and Visser, 1980). Ethylene, which promotes pistil development and delays staminate flower formation, appears to be the primary hormonal factor affecting sex expression, with other hormones acting through ethylene (Byers et al., 1972; Papadopoulou et al., 2005; Karakaya and Padem, 2011). Generally, the hormones involved in melon sex expression and the genetic pathway controlling melon sex determination have been determined. However, how the sex determination signals are perceived and how the information is translated to cause organ-specific abortion are still unclear. The multifactorial epigenetic regulation of sex determination is still poorly characterized.
Application of exogenous ethylene at the appropriate time can cause floral primordia destined to bisexual/male flowers altered to female flowers. The extent of conversion can be affected by genotype, stage of floral development at the time of application, and amount of applied hormone. To study the role of ethylene in sex expression and flower development, three inbred lines of melon characterized by differences in their flower types were used. The capabilities of ethrel (an ethylene releasing agent), AVG (an inhibitor of ethylene biosynthesis), and AgNO3 (an inhibitor of ethylene action) treatments to control sex expression and flower development in different melon sex types were analyzed. The main conclusion provides a basis for developing an exogenous hormone application method in melon hybrid seed production.
Materials and Methods
Plant Material and Growing Conditions
The experiments were carried out in an experimental field at Zhejiang University, Hangzhou China (30°14'45.08" N, 20°10'41.70" E, and 15.90 m above sea level) during the autumn of 2017. Three inbred lines of melon (produced by more than 10 generations of selfing) belonging to different genotypes and carrying different flower types (Fig. 1) were used as materials in this study. Andromonoecious C. melo var. cantaloupensis Naud. ‘PX’ was obtained by selfing and selecting from a commercial cultivar ‘Xuelihong’, monoecious C. melo var. cantaloupensis Naud. ‘PA’ was derived from the crossing and backcrossing of commercial cultivars ‘AM’ (monoecious) and ‘FengmiNo. 6’ (recurrent parent), and hermaphroditic C. melo var. flexuosus Makino ‘PB’ was produced from a traditional melon cultivar ‘Balengcui’ for pickling in China.
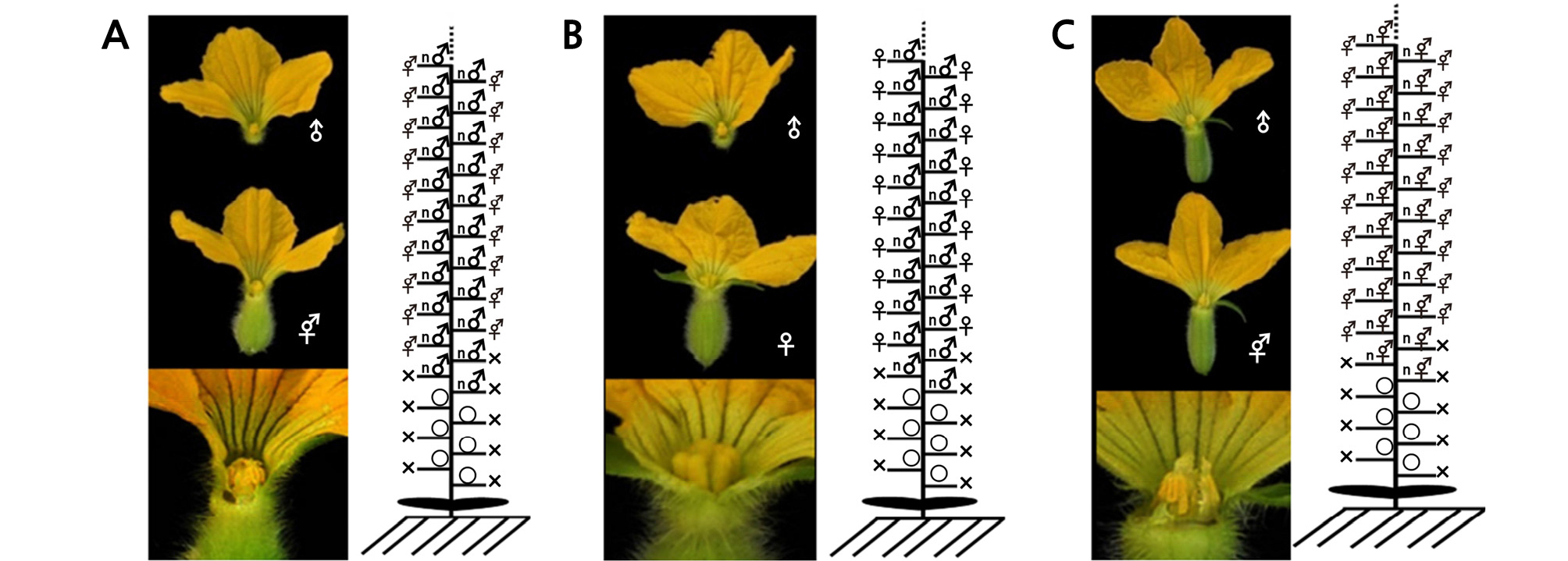
Fig. 1.
Flower characteristics and schematic representations of sex expression in andromonoecious ‘PX’ (A), monoecious ‘PA’ (B), and hermaphroditic ‘PB’ (C). Vertical lines indicate main stems, horizontal bars indicate lateral branches on the main stem, “ο” indicates no flower on the main stem, “n” indicates more than one flower, “×” indicates lateral branch removal, “♂” indicates male flower, “♀” indicates female flower, and “⚥” indicates bisexual flower.
Melon seeds were germinated on wet filter paper in Petri dishes for 1 day and then sown individually to 72-hole seedling trays on July 30, 2017. Plants were transplanted to the field at a spacing of 0.5 m × 0.6 m in a greenhouse on August 13 and pruned to maintain only one vine. The lateral branches between nodes 1 and 9 and higher than node 15 were removed, and the shoots of the remaining lateral branches were pinched between the 2nd and 3rd leaves (following the commercial practice). The other agricultural management procedures, such as irrigation, fertilizer management, and pathogen pest control, were performed according to standard management practices.
Chemical Treatments and Evaluation of Sexual Expression
The experiments were performed in three replications using a randomized complete block design. There were 210 plants within each sex type. To study the effects of ethylene on the sexual determination and development of male and female flowers in melon, the following treatments were carried out: E40, 100 µL·L-1 40% ethrel; E80, 200 µL·L-1 40% ethrel; E160, 400 µL·L-1 40% ethrel; SN + E80, 300 mg·L-1 silver nitrate (AgNO3)+ 200 µL·L-1 40% ethrel (300 mg·L-1 AgNO3 was sprayed and then 2 h later 200 µL·L-1 40% ethrel was sprayed); AVG, 100 mg·L-1 aminoethoxyvinylglycine (AVG); and SN, 300 mg·L-1 AgNO3. The treatments were applied twice to the shoot apices of all plants at the three-leaf stage (i.e., when the leaf blade of the third leaf was approximately 2 cm long) and at the six-leaf stage. Control plants were sprayed with clean water. The numbers and sex types of open flowers at each node in each plant were recorded daily after the first flower opened. The number of male, female, bisexual, and aborted flowers on the main stem; the number of female, bisexual, and aborted flowers on the lateral branches; the number of first male and bisexual flowers on the main stem; and the percentage of female, bisexual, male, and aborted flowers on the main stem and on the lateral branches, at each node per plant up to node 25, were calculated at the end of the experiment.
The statistically significant differences in floral development were determined by least significant difference tests and Duncan’s multiple comparisons.
Results
Effects of Ethrel, AVG, and AgNO3 on the Floral Development of Different Melon Genotypes
The control plants of andromonoecious ‘PX’ had male flowers on the main stem successively from nodes 5 ‑ 7 (mean 5.3) and had bisexual flowers on the lateral branch from node 9. The control plants of monoecious ‘PA’ had male flowers on the main stem successively from nodes 5 ‑ 7 (mean 5.2) and had female flowers on the lateral branch from node 10. The control plants of hermaphroditic ‘PB’ had only bisexual flowers on the main stem from nodes 5 to 6 (mean 5.7) and on the lateral branch from node 10 (Fig. 1). These three forms of melon had been previously deduced as “AAGGmmGyGy”, “AAGGMMGyGy ”, and “aaggmmgygy”, respectively.
Compared with the control, application of 40% ethrel inhibited the growth and development of the flowers. The ethrel treatment initially caused the severe stunting of the plants, but the leaf number did not decrease. The effect was more pronounced as the ethrel concentration increased. Later, the plants recovered and initiated new growth. The ethrel treatment also inhibited the occurrence of lateral branches. As the ethrel concentration increased, the inhibition of plant flower development significantly increased. Moreover, the ethrel-treated hermaphroditic ‘PB’ plants carried only one flower at each node on the main stem between nodes 10 and 15, while the control plants carried more than one flower at each node in the same region of the main stem.
The ethrel treatment resulted in the first flower of the main stem occurring on a much higher node, and all of the ethrel-treated plants showed a significant decrease in the numbers of flowers (Table 1). The nodes having the first flowers on the main stems were 1.9 ‑ 2.9, 1.6 ‑ 3.8, and 1.3 ‑ 2.0 times higher than that of the control and the numbers of nodes with flowers on the main stem were 44.0% ‑ 63.8%, 37.2 ‑ 84.2%, and 10.9 ‑ 36.8% less than that of the control in the andromonoecious ‘PX’, monoecious ‘PA’, and hermaphroditic ‘PB’ plants, respectively. The number of aborted flowers on the lateral branches between nodes 10 and 15 significantly increased as the ethrel concentration increased. Additionally, no open flowers were found on the lateral branches of andromonoecious ‘PX’ plants treated with 400 µL·L-1 40% ethrel or on monoecious ‘PA’ plants treated with 200 and 400 µL·L-1 40% ethrel. Thus, the effects of ethylene on floral development differed among the genotypes, with the monoecious ‘PA’ being the most affected and the hermaphroditic ‘PB’ being the least affected.
Table 1.
Effects of ethrel, AVG, and AgNO3 on melon traits related to floral development compared with the control
When AgNO3 and 40% ethrel (200 µL·L-1) were applied in combination, the first flower appeared at a much higher node on the main stem than the control, except in ‘PA’, and a significant decrease in the number of flowers occurred in all three cultivars. The effects of the combined treatment were similar to those of 40% ethrel alone using a concentration between 100 µL·L-1 and 200 µL·L-1 40% ethrel (i.e., stronger than the former, but weaker than the latter).
Applications of AVG or AgNO3 induced earlier flowers on the main stems in all three tested genotypes, and all of the flowers appeared successively along with the main stem. All of the plants responded in similar ways to AVG and AgNO3 treatments (Table 1). AVG and AgNO3 treatments promoted higher flower production levels on the main stem. Furthermore, in the andromonoecious ‘PX’ and the monoecious ‘PA’ plants, AgNO3 and AVG treatments resulted in the lateral branches having clusters of male flowers at the lower nodes, where no flowers appeared in the control plants. However, the AgNO3 and AVG treatments did not alter flower bearing on the lateral branches in the region where the control had carpel-bearing flowers. In the AVG- and AgNO3-treated hermaphroditic ‘PB’ plants, the earlier appearance of bisexual open flowers on the main stem resulted in earlier fruit setting than in the control (Fig. 2).
No significant differences were detected between the AVG and AgNO3 treatments for promoting earlier flowers. In the AVG- and AgNO3-treated andromonoecious ‘PX’ plants, the numbers of nodes with flowers on the main stem were 14.4% and 16.1% higher, respectively, than that of the control. In the AVG- and AgNO3-treated monoecious ‘PA’ plants, the numbers of nodes with flowers on the main stem were 16.0% and 17.3% higher, respectively, than that of the control. Additionally, AVG and AgNO3 promoted the initiation of bisexual flower production on the main stem in hermaphroditic ‘PB’ plants by 23.7% and 23.3%, respectively, compared with the control. The increases in the numbers of nodes with flowers caused by AVG and AgNO3 in the hermaphroditic ‘PB’ plants were greater than in the andromonoecious ‘PX’ and monoecious ‘PA’ plants.
Effects of Ethrel on Sex Expression in Different Melon Genotypes
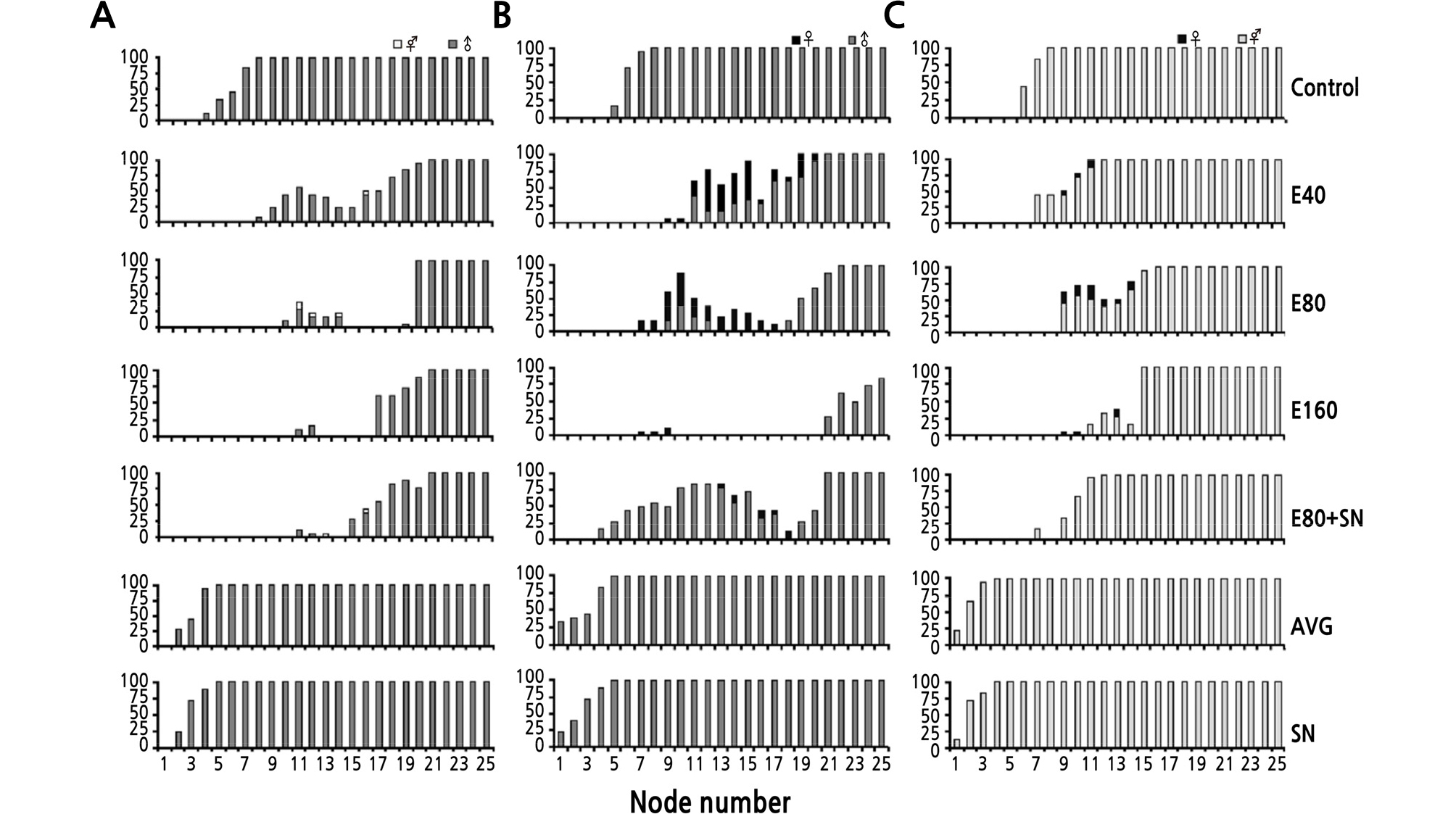
Applications of various concentrations of 40% ethrel (100, 200, and 400 µL·L-1) delayed the appearance of male and bisexual flowers on the main stem and inhibited the formation of female and bisexual flowers on the lateral branches. Moreover, ethrel altered the flower sex on the main stem (Fig. 3). In the andromonoecious ‘PX’ plants, 1, 4, and 2 bisexual flowers were detected on the main stems after 100 µL·L-1 40% ethrel, 200 µL·L-1 40% ethrel, and 300 mg·L-1 AgNO3 + 200 µL·L-1 40% ethrel treatments, respectively, while the control carried only male flowers on the main stem. However, the ethrel treatment failed to induce female flowers in the andromonoecious ‘PX’ plants (Fig. 3A). In the monoecious ‘PA’ plants, which normally produced only male flowers on the main stem, there were 55, 52, 4, and 8 female flowers on the main stems of the plants treated with the 100 µL·L-1 40% ethrel, 200 µL·L-1 40% ethrel, 400 µL·L-1 40% ethrel, and 300 mg·L-1 AgNO3 + 200 µL·L-1 40% ethrel, respectively (Fig. 3B). In the hermaphroditic ‘PB’ plants, there were 4, 15, and 4 female flowers in the 100 µL·L-1 40% ethrel, 200 µL·L-1 40% ethrel, and 400 µL·L-1 40% ethrel-treated plants, respectively. No female flowers were induced in the hermaphroditic ‘PB’ plants treated with 300 mg·L-1 AgNO3 + 200 µL·L-1 40% ethrel (Fig. 3C). In summary, the 200 µL·L-1 40% ethrel treatment was the most effective in changing the flower sex in andromonoecious ‘PX’ and hermaphroditic ‘PB’ plants, and the 100 µL·L-1 40% ethrel treatment had the greatest effect on the sex type transition in monoecious ‘PA’. Thus, the monoecious line ‘PA’ was the most sensitive to ethrel in terms of sex modifications among the three analyzed genotypes.
The major effects of ethrel on flower sex type and the number of nodes with flowers on the main stem occurred between nodes 9 and 15 and then decreased from node 16 to 21 compared with the control. The response region expanded as the ethrel concentration increased. Most of the flower sex type transitions were found between nodes 10 and 15, and the number of aborted lateral branches between nodes 10 and 15 significantly increased as the ethrel concentration increased (Fig. 4). In the andromonoecious ‘PX’ plants treated with 100 and 200 µL·L-1 40% ethrel, the ratios of male/ carpel-bearing flowers between nodes 10 and 15 were ~14% and 23% greater than that of the control. However, the ratio of male/carpel-bearing flowers in the AgNO3 plus ethrel-treated plants was 1-fold lower than that of the control. On the contrary, in the monoecious ‘PA’ plants treated with either 200 or 400 µL·L-1 40% ethrel, the ratio of male/carpel-bearing flowers was 58% lower than that of the control. However, the ratio of male/carpel-bearing flowers in the AgNO3 plus ethrel-treated plants was 2.4 times greater than that of the control. In the hermaphrodite ‘PB’ plants, the female flowers accounted for 1.5%, 7.8%, and 6.4% of the total flowers produced after applications of 100, 200, and 400 µL·L-1 40% ethrel, respectively.
The application of AVG or AgNO3 promoted flower production, but the AVG and AgNO3 treatments did not alter the sex types in the three analyzed melon cultivars. Although the number of carpel-bearing flowers did not decrease, and may have even increased, the ratio of male/carpel-bearing flowers was still greater than that of the control. This resulted from the large number of male flowers induced at the lower nodes in the AVG- and AgNO3-treated andromonoecious ‘PX’ and monoecious ‘PA’plants.
Discussion
In melon, floral primordia are initially bisexual with sex determination occurring by the selective developmental arrest of either the stamen or the carpel primordia, resulting in unisexual flowers. In buds that become female, increased ethylene produced by the andromonoecious gene in the carpel primordia leads to the arrest of stamen primordia (Boualem et al., 2008). The expression of the gynoecious gene suppresses carpel development and indirectly promotes the development of stamens by repressing the expression of the andromonoecious gene (Boualem et al., 2008; Martin et al., 2009). Additionally, the androecy gene represses the expression of the gynoecious gene to control the development and coexistence of male and female flowers in monoecious plants (Boualem et al., 2015; Ma and Pannell, 2016). However, it is still unknown how the male and female flowers coexist on the same plant. In monoecious plants, female flowers develop on the branches because of expression of the androecy gene that represses the expression of the gynoecious gene, but in male flowers developing on the main stem, the same androecy gene permits the expression of the gynoecious gene here. Therefore, what decides whether the androecy gene is on or off in particular flowers? Maybe an upstream regulatory cue (perhaps a hormone) turns the androecy gene on or off in different parts of monoecious plants (Boualem et al., 2016; Ma and Pannell, 2016).
The developmental fates of male and female organs are affected by a combination of the amount and sensitivity to ethylene (Yin and Quinn, 1992; Yin and Quinn, 1995; Zhang and Luan, 2015). Differing sensitivity levels in the stamen or carpel primordia could result in responses to different ranges of hormone concentrations and could inhibit or promote each sex independently. In the hermaphroditic ‘PB’ plants, the buds on the main stem that were destined to be bisexual were altered to female flowers by the ethrel treatment. In the ethrel-treated andromonoecious ‘PX’ and monoecious ‘PA’ plants, most of the carpel-bearing flowers on the lateral branches and a few of the male flowers on the main stem were aborted, and the abortion rate increased as the ethrel concentration increased. However, a few of the male flowers on the main stem were converted to bisexual flowers in the ethrel-treated andromonoecious ‘PX’ plants. Additionally, several buds on the main stem that were destined to be male flowers were converted to female flowers in the ethrel-treated monoecious ‘PA’ plants. However, why were the buds destined to be male flowers on the main stem converted to bisexual flowers in the ethrel-treated andromonoecious ‘PX’ plants instead of female flowers, like in the hermaphroditic ‘PB’ and monoecious ‘PA’ plants? More research is needed to confirm the mechanism of exogenous ethylene-induced sex alteration.
Exogenous application of ethylene-releasing chemicals leads to increases in femaleness in melon as manifested by the increased production of carpel-bearing flowers (i.e., bisexual or female) and/or the increased ratio of female flowers relative to bisexual flowers (Karchi, 1970; Girek et al., 2013). The present study, using 40% ethrel as an ethylene source, confirmed the feminizing effect of ethylene in melon. Meanwhile, applications of various concentrations of 40% ethrel delayed the appearance of male or bisexual flowers and inhibited the formation of flowers, resulting in flower loss, not only of male flowers but also the carpel-bearing flowers. Different genotypes differed in their sensitivity to ethylene, with the monoecious plants being the most sensitive followed by andromonoecious plants, and hermaphroditic plants being the most insensitive in this study. Stankovic et al. (2001) also found that ethrel treatments at the three-leaf stage affected melon sex expression and delayed the flowering process. In their research, the appearance of female flowers was delayed by ~10 days and that of male flowers was delayed more than 30 days compared with the control. In addition, ethrel was more effective in monoecious plants than in andromonoecious plants (Stankovic et al., 2001). However, some of previous reports that ethrel application resulted in an increase of the number of bisexual flowers and an earlier appearance of carpel-bearing flowers were not in agreement with our results (Karchi, 1970; Girek et al., 2013). The different results may be caused by using different genotypes, different pruning methods, different stages of floral development at the time of application, or different amounts of applied hormone.
AgNO3 and AVG have masculinizing effects on many dioecious and monoecious plants, and they act as anti-ethylene agents, inducing male flower production by suppressing female reproductive organs. AgNO3 and AVG can modify flower sex through the inhibition of ethylene (Karakaya and Padem, 2011; Manzano et al., 2014). In our experiment, AVG and AgNO3 treatments did not alter the sex of flowers and resulted in the opposite effects on flower development compared with ethrel treatments. AVG and AgNO3 treatments resulted in the first flower appearing on a lower node and increased the number of flowers on the main stem compared with the controls. AVG and AgNO3 treatments resulted in the earlier appearance of bisexual flowers in the hermaphroditic ‘PB’ plants, which led to earlier fruit set in the plants treated with AVG or AgNO3 than in the untreated plants. Unlike ethrel, AVG and AgNO3 had strong effects in the hermaphroditic plants compared with the andromonoecious and monoecious plants. The AgNO3 plus ethrel combination treatment indicated that ethrel had greater effects on the investigated traits than AgNO3, and there was an extinction effect between ethrel and AgNO3.
In conclusion, the ethrel treatment could change the three flower sex types on the main stem of the tested genotypes, delay the initiation of male and bisexual flowers on the main stem, and inhibit the formation of the lateral branches bearing female and/or bisexual flowers. The floral development and sex transition in the three sex types of the melons were affected differently in response to ethrel, with the monoecious line being the most sensitive and the hermaphroditic line being the most insensitive. The AVG and AgNO3 treatments resulted in the earlier initiation of bisexual flowers on the hermaphroditic line on the main stem. Thus, the external application of ethrel could be useful in melon hybrid seed production or in developing non-pruning technologies for melon, and the application of AVG and AgNO3 promoted earlier fruit setting in hermaphroditic melon. Studies with exogenous ethylene have indicated that the application timing and concentration are key factors to determine whether carpel or stamen development is affected (Switzenberg et al., 2014). The extent of sex conversion can be affected by genotype, floral developmental stage at the time of application, and the amount of the applied hormone. An increased understanding of application timing, times, hormone concentrations, and the corresponding pruning method is necessary to meet the demands for inducing unisexual flowers and promoting production.