Introduction
Materials and Methods
Materials
Isolation of Phenolic Compounds
Determination of Total Phenolic Content
Inhibitory Effect on 1,1-diphenyl-2-picrylhydrazyl (DPPH) Radicals
Inhibitory Effect on 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Radicals
Inhibitory Effect on Antioxidant Protection Factor (PF)
Inhibitory Effect on Thiobarbituric Acid Reactive Substances (TBARs)
Inhibitory Effect of Hyaluronidase (HAase)
Inhibitory Effect on Elastase
Inhibitory Effect on Collagenase
Inhibitory Effect on α-amylase
Inhibitory Effect on α-glucosidase
Statistical Analysis
Results and Discussion
Comparison of the Contents of Phenolic Compounds of the Peel and Whole Extracts of ‘Summer King’ Apples
Comparison of the Physicochemical Activities of Solid and Phenolic Compounds from Peel and Whole-Apple Extracts
Comparing Phenolic Compound Yields by Solvent Type and Concentration
Antioxidant Effects
Anti-Inflammatory (Hyaluronidase Inhibitory) Effects
Wrinkle Improvement (Elastase and Collagenase Inhibition) Effects
Anti-Diabetic (α-amylase and α-gulcosidase Inhibition) Effects
Conclusion
Introduction
Recently, products packaged in smaller units have been gaining popularity due to the increasing number of single-person households. Because of this, the demand for small varieties of apples (Malus domestica) has been increasing. According to the recently published ‘2018 Agricultural Outlook’, the demand for small varieties of apples (less than 213 g) for household consumption has doubled from 8.7% in 2014 to 16.6% in 2017. However, the demand for larger varieties of apples (over 300 g) has dropped from 9.4% to 3.9% over the same time period. Moreover, according to a report from the Rural Economic Institute, the prices of small- and middle-sized varieties of apples has risen bt 9 ‑ 22% compared to larger varieties of apples (KREI, 2018).
Apples are a temperate zone fruit tree belonging to the Rosaceae family, and they account for 40% of the total fruit growing area in Korea. Apples contain polysaccharides, plant sterols, phenols, proteins, vitamins, and essential trace elements crucial for human health. In particular, the antioxidant content of the apple peel is 2 ‑ 9 times higher than that found in the pulp (Wolfe and Liu, 2003; Li et al., 2006; Feliciano et al., 2010). Polyphenols are the major antioxidants found in apples (Eberhardt et al., 2000; Lee et al., 2003), and this class of compounds has demonstrated anti-inflammatory and antitumor effects, and proven benefits for human health (Jedrychowski et al., 2009; Sul et al., 2009; Hwang et al., 2011; Baranowska-Wójcik and Szwajgier, 2019).
Evaluation of extracts from Green ball apple, a new breed, and apples of ‘Fuji’ cultivar grown in Korea also showed inhibitory effects of enzymes involved in whitening, wrinkle improvement, and anti-inflammatory properties (Lee et al., 2018). Also, the polyphenolic compounds in apples (procyanidin, chlorogenic acid, caffeic acid, epicatechin, catechin, p-coumaroyl qunic acid, rutin, phloridzin, quercetin, etc.) are known to have diverse beneficial physiological activities in humans, including anti-oxidant, anti-allergic, anti-diabetic, anticancer, anti-inflammatory, skin whitening, and anti-wrinkle effects (Li et al., 2006; Sul et al., 2009; Henríquez et al., 2010; Supapvanich et al., 2018). Owing to its antioxidant potential with low toxicity, several articles have assessed its use as an active extract of cosmetic ingredients.
These phenolic compounds are highly concentrated in the apple pericarp, and their antioxidant and physiological activities have been reported to be superior when derived from the peel, which supports the increasing interest in the function and application of fruit skin (Wolfe et al., 2003; Henríquez et al., 2010; Kubola and Siriamornpun, 2011). Malus domestica Borkh is a new cultivar of apple called ‘Summer King’, which is a hybrid of the ‘Fuji’ and ‘Golden delicious’ cultivars. It is a small, red apple with a weight of about 265 g, and is harvested in August.
In this study, we investigated the various physiological properties of ‘Summer King’ apples in an effort to highlight this fruit as a high value functional food source.
Materials and Methods
Materials
‘Summer King’ (Malus domestica Borkh) apple, a newly-bred cultivar cultivated by crossbreeding at Apple Research Institute located at Gunwi, Gyeongsangbuk-do, is the product of a cross between ‘Fuji’ and ‘Golden delicious’. ‘Fuji’ apples were used as the control material in this study. The apples were harvested on August 6, and the fruit peel and the whole apple were used for testing. The apple samples were dried in a freeze dryer (FD8518, Ilshin Biobase, Yangju, Korea) and subsequently pulverized using a 40 mesh and stored at 4°C for the analyses.
Isolation of Phenolic Compounds
Two different extraction methods were used (water and ethanol extraction). To prepare the water extract, 1 g of fruit powder was immersed in 200 mL of distilled water, boiled until the total volume was reduced to 100 mL, cooled, and stirred for 24 h. To prepare the ethanol extract, 1 g of fruit powder was mixed with 100 mL of 10 ‑ 100% ethanol and stored at 4°C for 24 h with shaking. Water and ethanol extracts were filtered through Whatman No. 1 filter paper (Whatman Inc., Piscataway, NJ, USA), concentrated by using a rotary vacuum evaporator (Eyela NE, Tokyo, Japan), and stored at 4°C until use. The concentrations of phenolic compounds in the water- and ethanol-extracted samples were tested after dilution into 25, 50, 75, and 100 µg·mL-1 or 50, 100, 150, and 200 µg·mL-1, respectively, for further experiments.
Determination of Total Phenolic Content
Total phenolic contents of the water and ethanol extracts were measured by using the method described by Folin and Denis (1912). Briefly, 1 mL of the extract was added to 6.5 mL of solution A, containing 1 mL of 95% ethanol, 5 mL of distilled water, and 0.5 mL of 1N Folin–Ciocalteu reagent; this was followed by a 5 min incubation at room temperature. The absorbance was measured at 725 nm within 1 h after the addition of 1 mL of 5% Na2CO3. The total phenolic compound content was calculated by using the standard curve of gallic acid.
Inhibitory Effect on 1,1-diphenyl-2-picrylhydrazyl (DPPH) Radicals
The free radical-scavenging potential of apple extracts was measured as previously described (Blois, 1958). Three mL of 60 µM 1,1-diphenyl-2-picrylhydrazyl (DPPH) was added to 0.5 mL of samples and mixed by vortexing. These mixtures were stored at room temperature for 15 min. The absorbance was measured at 517 nm for the DPPH radical- scavenging effect of apple extracts.
Inhibitory Effect on 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Radicals
ABTS radical inhibition was measured as previously described by Pellegrini et al. (1998). A solution containing 7 mM of ABTS and 140 mM K2S2O8 was mixed with 88 µL of 5 mL and left in the dark for 12 to 16 h. This solution was then mixed with ethanol at a ratio of 1:88, and the solution was adjusted to an absorbance of 0.7 ± 0.02 at 734 nm. A total of 50 µL of the sample solution and 1 mL of the ABTS solution were mixed for 30 seconds, and incubated for 2.5 min. The absorbance was measured at 734 nm for ABTS activity of apple extracts, and the difference between the control and ‘Summer King’ samples was used to calculate the ABTS cation radical decolorization value.
Inhibitory Effect on Antioxidant Protection Factor (PF)
PF measurements were carried out according to a previously published method by Andarwulan and Shetty (1999). In brief, 10 mL of β-carotene was dissolved in 50 mL of chloroform, and 1 mL of the solution was added to an evaporator. The chloroform was distilled off in a water bath at 40°C. Subsequently, 20 µL linoleic acid, 184 µL Tween 40, and 50 mL H2O2 were added to make an emulsion. In total, 5 mL of the emulsion was mixed with 100 µL of the test sample, vortexed, and allowed to react at 50°C for 30 min. The absorbance was measured at 470 nm, and the difference between the control and ‘Summer King’ was used to calculate the PF value.
Inhibitory Effect on Thiobarbituric Acid Reactive Substances (TBARs)
The inhibitory effect of TBARs was measured by mixing 0.8 mL of emulsion and 0.2 mL of dissolved sample in 1% linoleic acid solution and 1% Tween 40 according to a previously published method of Buege and Aust (1978). In total, 2 mL of TBA regent was added to 1 mL of reaction solution, boiled for 15 min, cooled for 10 min, centrifuged at 1,000 rpm for 15 min, and incubated at room temperature for 10 min. The supernatant was then taken, and its absorbance was measured at 532 nm. TBAR values were calculated by subtracting the absorbance observed from the control samples from the absorbance of one of the ‘Summer King’ samples. The control samples were prepared by using distilled water instead of extraction samples.
Inhibitory Effect of Hyaluronidase (HAase)
The inhibitory effect of HAase was measured by using the Dorfman and Ott (1948). N-acetyl-glucosamine formed from sodium-hyaluronic acid (HA) was transformed into a glucoxazoline derivative. Subsequently, the absorbance was measured at 600 nm by coloring with ρ-dimethyl-aminobenzaldehyde (DMAB).
Inhibitory Effect on Elastase
The inhibitory effect on elastase was assessed using a previously published method by Kraunsoe et al. (1996). In total, 1.0 U/mL of porcine pancreatic elastase (PPE) (Sigma-Aldrich Co.) was added to 1 mL of 0.2 M Tris-HCl buffer (pH 8.0) in 0.1 mL of a 0.8 mM N-succinyl- (Ala) enzyme solution and 0.1 mL of 50 to 200 µg/mL phenolics was added. In the control reaction, 0.1 mL of distilled water was added instead of the sample. The reaction was conducted at 25°C for 20 min, and the amount of ρ-nitroaniline produced was measured at an absorbance of 410 nm. The difference between experimental and control samples was calculated.
Inhibitory Effect on Collagenase
Collagenase inhibition was measured using the method of Wunsch and Heidrich (1963). A total of 0.25 mL of a substrate solution, in which 4 mM CaCl2 and 0.3 mg/mL 4-phenylazobenzyloxycarbonyl-Pro-Leu-Gly-Pro-D-Arg were dissolved in 0.1 M Tris-HCl buffer (pH 7.5), and 0.15 mL of 0.2 mg/mL collagenase (Sigma-Aldrich Co.) was added to 0.1 mL of each sample solution at a concentration of 50 ‑ 200 µg·mL-1 phenolic compounds. After incubation at room temperature for 20 min, 0.5 mL of 6% citric acid was added. After the reaction was stopped, 2 mL of ethyl acetate was added, and the absorbance was measured at 320 nm.
Inhibitory Effect on α-amylase
The inhibitory effect on α-amylase was determined according to a previously published method of Davidson and Parish (1989). In total, 5 g of agar and 5 g of soluble starch were dissolved in distilled water and boiled. The solution was sterilized at 121°C for 15 min and then poured into a Petri dish in 15 mL increments. After that, a 10 mm diameter disc paper was placed on top, and 0.8 mL of the sample solution and 0.2 mL of the enzyme solution (1,000 U/mL) were mixed and dispensed onto the disc paper. In the control reaction, distilled water was added instead of the sample. The reactions were incubated at 37°C for 3 days, and 3 mL of I2/KI (5 mM I2 in 3% KI) was then added. After 15 min of color development, the clear zone was calculated, and the inhibition rate was measured.
Inhibitory Effect on α-glucosidase
The inhibitory effect on α-glucosidase was measured according to the method of Tibbot and Skadsen (1996). p-Nitrophenol-α-D-glucopyranoside (PNPG) was dissolved in 50 mM sodium succinate buffer (pH 6.8) to generate a substrate with a concentration of 1 mg/mL. In total, 1 mL of the substrate and 0.1 mL of the enzyme solution were mixed, then 0.1 mL of distilled water was added to the control and 0.1 mL of the 200 µg/mL sample was added to the reaction mixture. The mixtures were reacted at 37°C for 30 min. Finally, 0.1 mL of 1 N NaOH was added to develop the color, and the absorbance was measured at 400 nm. Enzyme reaction with distilled water instead of samples was used as a control.
Statistical Analysis
All tests were repeated at least three times. Statistical analysis (mean ± standard deviation) was performed using SPSS 23 for Windows (Statistical Package for Social Science, Chicago, IL, USA). Analysis of variance with Duncan's multiple range test and one-way ANOVA were used to compare the significance of differences between the samples. The results were considered significant at p < 0.05 level.
Results and Discussion
Comparison of the Contents of Phenolic Compounds of the Peel and Whole Extracts of ‘Summer King’ Apples
To protect themselves from damage caused by oxidation or autoxidation by ultraviolet rays, plants produce antioxidants, including polyphenols, flavonoids, and acids. Phenolic compounds are known to have various physiological activities, such as antioxidant, anti-allergic, anti-diabetic, anti-cancer, anti-inflammatory, whitening, and wrinkle improvement activities (Lee et al., 2018; Park and Song, 2018). In recent years, many advancements have been achieved in detailing the physiological activities of substances derived from medicinal plant resources, and these substances have been proven to be efficacious as functional foods and cosmetics. As such, the development of functional foods and food ingredients is meaningful in terms of efficient utilization of natural resources, development of new food resources, and the development of new materials.
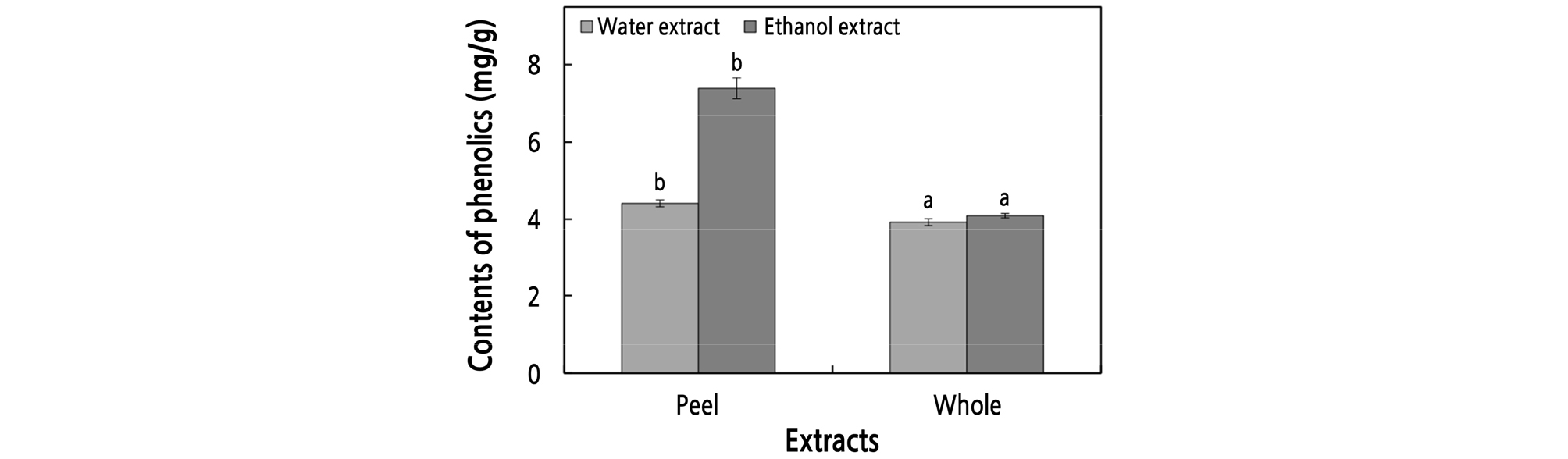
The peel and whole-apple samples of ‘Summer King’ apples were extracted with water and 60% ethanol, and their phenolic contents were measured as described above. As shown in Fig. 1, the highest phenolic content was found in the peel, which was about 2.26 times higher than that of whole-apple extracts, indicating that the physiological activity of the peel extract is the highest.
Comparison of the Physicochemical Activities of Solid and Phenolic Compounds from Peel and Whole-Apple Extracts
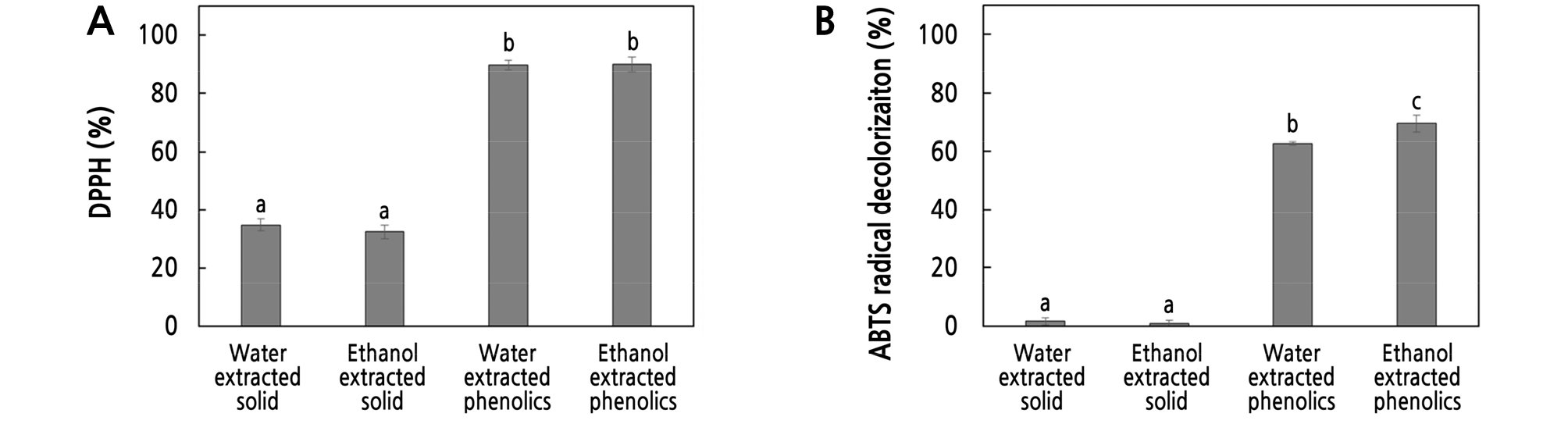
DPPH radicals were measured for comparison of the physiological activity of the solid and phenolic components of the apple samples. As shown in Fig. 2A, the solubility of the water and ethanol extracts were 34.69% and 32.35% at a phenolic concentration of 100 µg·mL-1, respectively. However, these samples demonstrated 89.57% and 89.83% DPPH radical scavenging ability, respectively, at a phenolic concentration of 100 µg·mL-1, respectively.
The ABTS radical scavenging activity of the samples was also measured. As shown in Fig. 2B, the water and ethanol extracts demonstrated very low electron donating abilities of 1.62% and 0.91%, respectively, at the solid content of 100 µg·mL-1. However, the ABTS radical decolorization activity was 62.67% and 69.55% at the phenolic concentration of 100 µg·mL-1. The biological activities of apples depend on their phenolic compounds rather than solid. Therefore, we speculated that the phenolic compounds in the apple peel were directly related to the observed physiological activity. Therefore, we extracted the phenolic compounds of the apple peels to examine their activity relative to their potential use in cosmetics and functional foods.
Comparing Phenolic Compound Yields by Solvent Type and Concentration
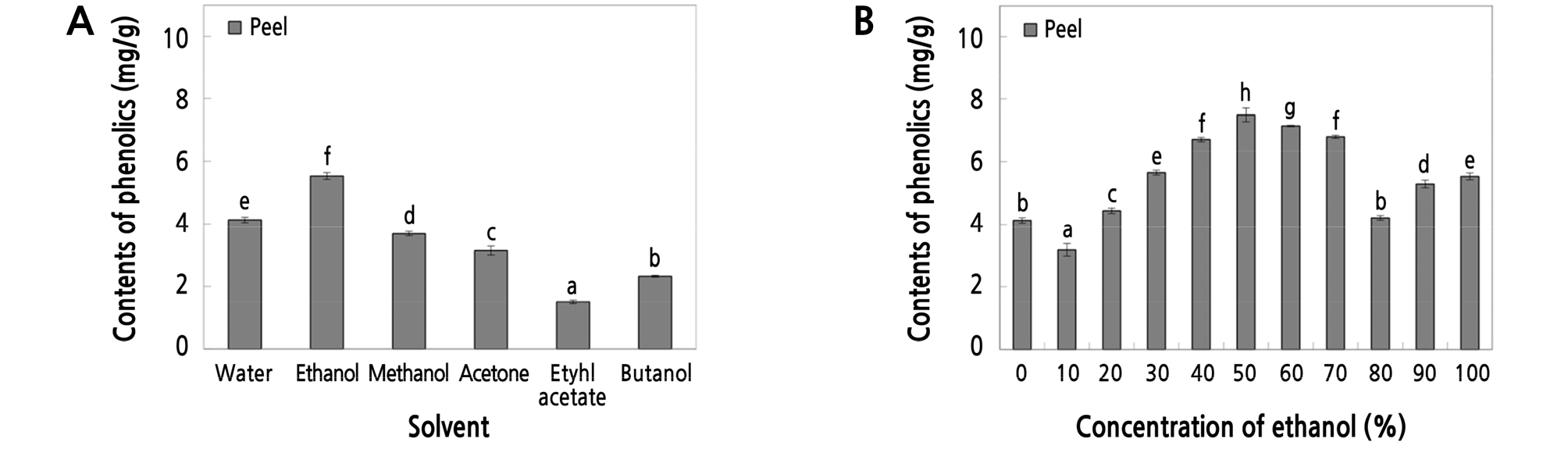
The solvent used for extraction can have a substantial influence on the concentration and activity of the isolated substances. Extraction with pure water is typically not harmful to the isolated compounds and can result in a high yield during the extraction of water-soluble substance. Ethanol is a polar solvent and is often used for extraction of useful components, including tannins, saponin, and organic acids.
We measured the phenolic contents of the apple peel and total apple extracts from ethanol, water, and organic solvents. As shown in Fig. 3A, the phenolic contents of the peel extracts were 5.86, 4.11, 3.96, 3.42, 2.66, and 1.84 mg·g-1 following extraction with ethanol, water, methanol, acetone, butanol, and ethyl acetate, respectively. In the peel extracts generated with ethanol, the highest yield of phenolic contents was 7.73 mg·g-1 when using 50% ethanol (Fig. 3B). Based on these results, extracts were prepared from peels and whole apples using water and 50% ethanol as the solvent, and the phenolic content of the water and 50% ethanol extracts was adjusted to 25 ‑ 200 µg·mL-1 for reproducibility of the subsequent experiments. These extracts were used to verify their functional activities.
Antioxidant Effects
Antioxidants neutralize free radicals that can be harmful to cells, including oxidative damage of DNA and lipids. Therefore, antioxidants are one of the primary physiologically active substances that are a current focus of research. Additionally, antioxidants are known to prevent the deterioration of food, prevent aging in the human body, and prevent a number of adult diseases (Farag et al., 1989; Frei, 1994). Antioxidants are highly reactive and protect cells by blocking active oxygen-induced chain reactions (Tibbot and Skadsen, 1996), and there are numerous antioxidant enzymes, including superoxide dismutase (SOD), catalase, glutathione peroxidase (GSH-Px), and glutathione S-transferase (GST), at work in the human body. Antioxidants and free radical scavengers, including vitamin E, β-carotin, carotenoids, flavonoids, and some minerals, such as selenium, are known to help protect the human body from oxidative damage (Borrello et al., 1984).
The DPPH radical scavenging activity of the peel extracts was measured to evaluate their anti-oxidative effects. As shown in Fig. 4A, the scavenging activity was 89.32 ‑ 90.97 and 90.16 ‑ 91.70% at a 25 ‑ 100 µg·mL-1 phenolic concentration in the water and ethanol peel extracts, respectively. The water and ethanol peel extracts and whole ‘Fuji’ apple extracts (used as a control) are shown in Fig. 4A and demonstrated 85.16 ‑ 87.55 and 87.80 ‑ 94.40% scavenging activity, respectively. These results indicated that the peel extracts possessed a better electron-donating ability at low concentrations relative to the whole ‘Fuji’ apple extract.
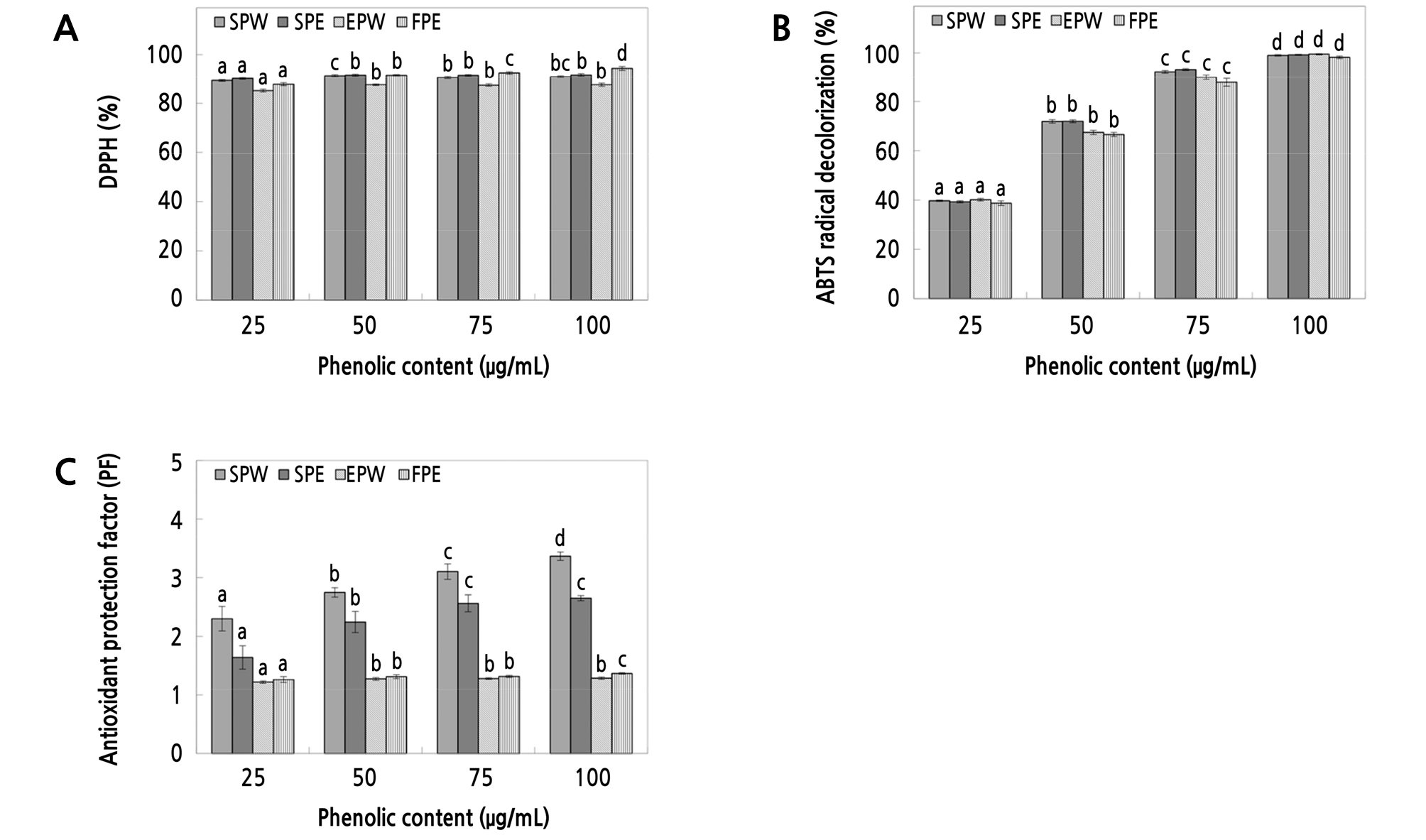
Fig. 4.
DPPH (A), ABTs (B), and antioxidant protection factor (C) of water and ethanol extracts from the peels of ‘Summer King’ apples. SPW: ‘Summer King’ apple peel water extract, SPE: ‘Summer King’ apple peel ethanol extract, FPW: ‘Fuji’ apple peel water extract, FPE: ‘Fuji’ apple peel ethanol extract. Means with different letters are significantly different at p < 0.05 based on Duncan’s multiple range tests.
The ABTS radical scavenging activity of the peel extracts was measured to help further evaluate their anti-oxidative effects. As shown in Fig. 4B, the scavenging activity was 39.70 ‑ 98.99 and 39.27 ‑ 99.21% with a 25 ‑ 100 µg·mL-1 phenolic concentration in the water and ethanol peel extracts, respectively. The water and ethanol peel extracts of peels and whole ‘Fuji’ apple extracts (used as a control) are shown in Fig. 4B and demonstrated 40.15 ‑ 99.46 and 38.70 ‑ 98.22% scavenging activity, respectively. These results indicated that the ‘Summer King’ apple peel extracts generally exhibited higher electron donating ability compared to the controls. Together, these results indicated the DPPH and ABTS radical scavenging ability was very high in the peel extracts.
β-carotene, which is composed of 11 double bonds and has a high degree of unsaturation, reacts very easily with peroxyl radicals. This compound also has the ability to perform selective cleavage of unsaturated carbons, thus acting as an antioxidant. To evaluate the anti-oxidative effects of the apple peel extracts, PF was measured using this property of β-carotene as an indicator of lipid-soluble antioxidant activity. As shown in Fig. 4C, the water and ethanol ‘Summer King’ peel extracts had 2.30 ‑ 3.37 PF and 1.64 ‑ 2.65 PF at a 25 ‑ 100 µg·mL-1 phenolic concentration, respectively. The results from the water and ethanol peel extracts and whole ‘Fuji’ apple extracts (control) are shown in Fig. 4C and were 1.22 ‑ 1.37 PF and 1.18 ‑ 1.41 PF, respectively, confirming that the ‘Summer King’ peel extracts possessed a superior lipid-soluble anti-oxidative ability compared to the controls. These results support the use of ‘Summer King’ apples as a functional material for anti-aging products.
Anti-Inflammatory (Hyaluronidase Inhibitory) Effects
Hyaluronic acid (HA) inhibits the phagocytic ability of macrophages, an important factor during the inflammation response, while HA degradation products or low molecular HA can increase inflammation, fibrosis, and collagen deposition during wound healing. In addition, hyaluronidase is generally present in an inactive form in lysosomes and helps to maintain homeostasis. However, HA is known as an inflammatory substance because it is activated during the development of inflammatory diseases, such as physical injury or rheumatism, and is involved in vascular permeability and inflammation reactions. It is also reported to be involved in allergic reactions and cancer cell metastasis (Ghosh, 1994).
The inhibitory effects of the peel extracts on hyaluronidase are shown in Fig. 5A. The water extract of ‘Summer King’ apple peel showed an inhibitory effect of 1.68 ‑ 4.97% at a phenolic concentration of 50 ‑ 200 µg·mL-1. The water extracts of the ‘Fuji’ apple peel are shown in Fig. 5A and demonstrated an inhibitory effect of 0.03 ‑ 1.79% at 50 ‑ 200 µg·mL-1. The ethanol extract of the ‘Summer King’ apple peel showed an inhibitory effect of 4.56 ‑ 20.40% at a 50 ‑ 200 µg·mL-1 phenolic concentration, and the inhibitory effect of the ethanol-extracted ‘Fuji’ apple peel at a phenolic concentration of 50 ‑ 200 µg·mL-1 was 1.29 ‑ 12.72%. The hyaluronidase inhibitory effects of the ‘Summer King’ apple extracts were clearly better than that of the ‘Fuji’ apple extracts, further supporting the application of ‘Summer King’ apple peel extracts in functional products utilizing their anti-inflammatory and atopic inhibitory effects.
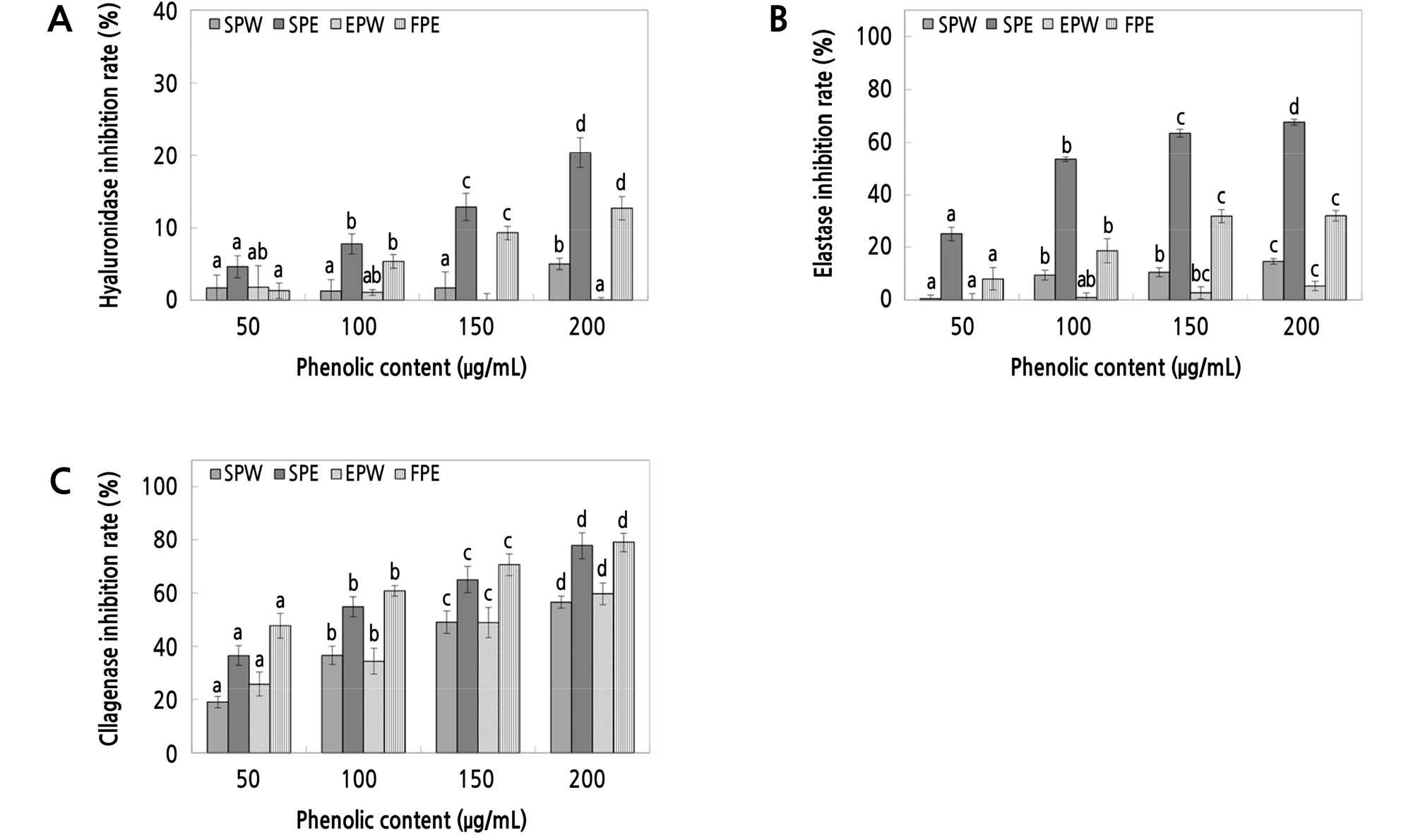
Fig. 5.
Inhibition activity of water and ethanol extracts from peels of ‘Summer King’ apples on hyaluronidase (A), elastase (B), and collagenase (C). SPW: ‘Summer King’ apple peel water extract, SPE: ‘Summer King’ apple peel ethanol extract, FPW: ‘Fuji’ apple peel water extract, FPE: ‘Fuji’ apple peel ethanol extract. Means with different letters are significantly different at p < 0.05 based on Duncan’s multiple range tests.
Wrinkle Improvement (Elastase and Collagenase Inhibition) Effects
The content of elastin, a structural protein of the skin, decreases as we age. The biosynthesis of type-1 collagenase is increased according to the degree of skin aging, thereby inducing degradation of matrix proteins, including collagen fibers and elastic fibers in the dermis, lowering skin elasticity and causing wrinkles (Lee et al., 2013). Elastase, present in the neutrophil granules of the human body, is an enzyme that decomposes elastin in the skin, and inhibition of elastase can improve the development of skin wrinkles (Lee and An, 2012).
The results of measuring the inhibitory effect of elastase are shown in Fig. 5B. The water extract of the ‘Summer King’ apple peel showed 0.58 ‑ 14.55% inhibition of elastase at a phenolic concentration of 50 ‑ 200 µg·mL-1. The water extracts of ‘Fuji’ apple peels are shown in Fig. 5B and demonstrated an inhibition of 0.00 ‑ 5.35% at 50 ‑ 200 µg·mL-1. The ethanol extract of the ‘Summer King’ apple peel had an inhibitory effect of 25.06 ‑ 67.73% at a 50 ‑ 200 µg·mL-1 phenolic concentration, and the inhibitory effect of the ethanol extract of ‘Fuji’ apple peels at a phenolic concentration of 50 ‑ 200 µg·mL-1 was 8.02 ‑ 32.10%. These results confirmed that the inhibitory effect on elastase of the ‘Summer King’ apple peel extracts was superior to that of the controls, supporting its use in functional cosmetics for treatment of wrinkles.
Collagen is a functional component of the skin that provides mechanical strength in addition to its roles in cell division and differentiation. Internal factors, such as decreased cell activity due to natural aging, and external factors, such as photo-aging due to ultraviolet irradiation, increase the stress experienced by the skin caused by various harmful environments. It has been reported that the reduction of collagen reduces the elasticity of the skin and causes the skin to wrinkle (Giacomoni and Rein, 2001).
The inhibitory effect on collagenase, an enzyme that degrades collagen, was assessed in the apple peel extracts, and the results are presented in Fig. 5C. The water extract of ‘Summer King’ apple peels showed 18.96 ‑ 56.60% inhibition at a phenolic concentration of 50 ‑ 200 µg·mL-1. The water extracts of ‘Fuji’ apple peels are shown in Fig. 5C and demonstrated inhibition of 25.89 ‑ 59.73% at 50 ‑ 200 µg·mL-1 phenolic concentration. The ethanol extract of ‘Summer King’ apple peels showed an inhibitory effect of 36.66 ‑ 77.81% at a 50 ‑ 200 µg·mL-1 phenolic concentration. The ethanol extract of ‘Fuji’ apple peels showed an inhibitory effect of 47.79 ‑ 79.05% at a 50 ‑ 200 µg·mL-1 phenolic concentration. The effect of the ‘Summer King’ apple extract was similar for each concentration interval. Therefore, these results confirmed that ‘Summer King’ apple peel extracts might have a positive effect on wrinkle reduction due to the ability to inhibit elastase and collagenase, enzymes that affect wrinkle induction and are important proteins involved in maintaining skin elasticity.
Anti-Diabetic (α-amylase and α-gulcosidase Inhibition) Effects
Starch is hydrolyzed by α-amylase in the intestine, and glucose is hydrolyzed by maltase in the small intestine, both of which are subsequently absorbed into the bloodstream, resulting in an increase of blood sugar levels and insulin levels. Therefore, controlling the activation of α-amylase can delay the digestion rate of starch and affect the distribution of its absorption kinetics. As such, it would be useful for patients with diabetes or obesity to control α-amylase activation, which could increase the therapeutic effects of specific diets (Brodbeck, 1980; Puls et al., 1997).
The results of measuring the inhibitory effect on α-amylase of ‘Summer King’ apple peel extracts are shown in Fig. 6A. The inhibitory effect was 13.55 ‑ 22.31% and 11.75 ‑ 26.77% at a phenolic concentration of 50 ‑ 200 µg·mL-1 for the water and ethanol extracts, respectively. The water and ethanol extracts of ‘Fuji’ apple peels were used as controls, and these results are shown in Fig. 6A, the inhibitory effect of these extracts were 1.19 ‑ 2.02% and 0.84 ‑ 0.91% at a phenolic concentration of 50 ‑ 200 µg·mL-1, respectively.
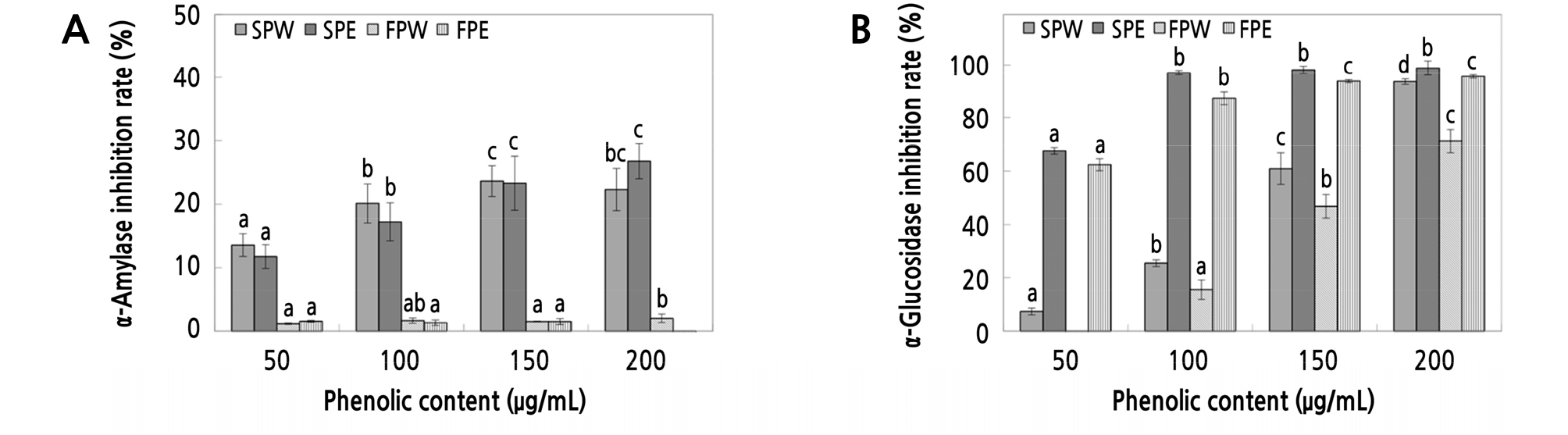
Fig. 6.
Inhibition activity of water and ethanol extracts from ‘Summer King’ apple peels and ‘Fuji’ apple peels on α-amylase (A) and α-glucosidase (B). SPW: ‘Summer King’ apple peel water extract, SPE: ‘Summer King’ apple peel ethanol extract, FPW: ‘Fuji’ apple peel water extract, FPE: ‘Fuji’ apple peel ethanol extract. Means with different letters are significantly different at p < 0.05 based on Duncan’s multiple range tests.
α-Glucosidase is present in the chorion of the small intestine, and this enzyme cleaves disaccharides and polysaccharides into smaller monosaccharides to accelerate the uptake of the sugars, which can result in hyperglycemia. In particular, the absorption of glucose is accelerated at 30 min after a meal, at which point blood glucose is increased. In patients with diabetes, the diabetic neuropathy, kidney disorder, and retinopathy are caused by rapidly increasing hyperoxaluria or hyperglycemia (McDougall and Stewart, 2005).
The inhibitory effect on yeast-derived α-glucosidase was measured in the apple extracts. As shown in Fig. 6B, the water extract of ‘Summer King’ apple peel showed a high inhibition effect of 7.39 ‑ 93.59% at a phenolic concentration of 50 ‑ 200 µg·mL-1. The ethanol extracts of ‘Summer King’ apple peel also showed a very high inhibitory effect of 67.63 ‑ 98.80% at a 50 ‑ 200 µg·mL-1 phenolic concentration. The results of the water and ethanol extracts of ‘Fuji’ apple peels are shown in Fig. 6B, indicating an inhibitory effect of 0.00 ‑ 63.33% and 62.41 ‑ 95.56% at a phenolic concentration of 50 ‑ 200 µg·mL-1, respectively. The ‘Summer King’ apple peel extracts were found to exhibit better inhibitory effects towards α-glucosidase at low concentrations relative to the ‘Fuji’ apple extracts. Therefore, these results confirmed that the ‘Summer King’ apple peel extracts strongly inhibited the activity of carbohydrase, suggesting that such extracts could be applied as an active component in functional foods.
As shown in the above results, the ‘Summer King’ apple has remarkably superior physiological activity compared to ‘Fuji’ apples and could be used in cosmetics and anti-diabetic foods due to their unique activities, including products aimed at anti-oxidation, wrinkle improvement, and anti-inflammation. The peel extracts showed very good activity; as such, it may be desirable to utilize the peels of these apples in functional applications directed at consumers.
Conclusion
After extracting the phenolic compounds from the ‘Summer King’ apple peel, we examined their functional properties to investigate the possibility of using this material in cosmetic and food products. When the phenolic compounds were extracted with water or ethanol, the phenolic contents were 4.41 and 7.39 mg·g-1, respectively. The anti-oxidant effects of ‘Summer King’ apple peel extracts were as follows: DPPH showed an inhibition of 89.32% and 90.16% at a 25 µg·mL-1 phenolic concentration in the water and ethanol extracts, respectively. ABTS demonstrated inhibition of 98.99% and 99.21% at a 100 µg·mL-1 phenolic concentration in the water and ethanol extracts, respectively. The anti-oxidant protection factor was determined to be very high at 3.37 PF and 2.65 PF at a 100 µg·mL-1 phenolic concentration in the water and ethanol extracts, respectively. These results support the use of these extracts as natural antioxidants for the prevention of aging. The hyaluronidase inhibitory effect of the ‘Summer King’ apple peel extracts was 4.97% and 20.40% at the phenolic concentration of 200 µg·mL-1 in the water and ethanol extracts, respectively. The elastase and collagenase inhibitory effects were 14.55% and 56.60%, respectively, in the water extract and 67.73% and 77.81%, respectively, in the ethanol extract, when measured at a 200 µg·mL-1 phenolic concentration. The α-amylase inhibitory effect of the water extract was 13.55 ‑ 22.31% at a 50 ‑ 200 µg·mL-1 phenolic concentration and 11.75 ‑ 26.77% in the ethanol extract at the same phenolic concentration. The ethanol extract showed a lower inhibitory effect; however, compared with the inhibitory effect of the ‘Fuji’ apple extracts (1.00 ‑ 2.02% for water and 0.84 ‑ 0.91% for ethanol) the ‘Summer King’ apple extracts showed a relatively high inhibitory effect. The inhibitory effect on yeast α-glucosidase activity was assessed at phenolic concentrations of 50 ‑ 200 µg·mL-1, and the water and ethanol extracts of the ‘Summer King’ apples showed very high inhibitory effects of 7.39 ‑ 93.59% and 67.63 ‑ 98.80%, respectively. The inhibitory effects of the control extracts were 0 ‑ 63.33% and 62.41 ‑ 95.56% at a 50 ‑ 200 µg·mL-1 phenolic concentration for the water and ethanol extracts, respectively. Previous studies on similar natural materials, such as Hericium erinaceus, Chionanthus retusa flower, and Cedrela sinensis fruits, have been reported. Analysis of the inhibition of hyaluronidase, elastase, collagenase, and α-glucosidase in these species showed an efficacy similar to that of ‘Summer King’ apple. Therefore, these materials could be developed as functional products as medicinal plant resources. Also, it can be concluded that the ‘Summer King’ apple peel extracts had a better inhibitory effect than the ‘Fuji’ apple extracts at low concentrations. Based to the above results, the anti-oxidative, anti-inflammatory, anti-diabetic, and wrinkle-reducing effects of the ‘Summer King’ apple peel extracts were superior to that of the ‘Fuji’ apple extracts. Together, these results suggest that components of the ‘Summer King’ apple could be used in cosmetics and functional food applications.