Introduction
Materials and Methods
Preparation of Seeds
Imbibition
Embryo Growth, Radicle Emergence, and Shoot Emergence in the Field
Effect of Temperature on Radicle Emergence
Cold Temperature Requirements for Shoot Emergence
Effect of GA3 Treatment on Radicle Emergence
Results
Imbibition
Embryo Growth, Radicle Emergence, and Shoot Emergence in the Field
Effect of Temperature Regimes on Radicle Emergence
Effect of Cold Temperature on Shoot Emergence
Effect of GA3 Treatment on Radicle Emergence
Discussion
Introduction
At the time of dispersal, seeds of many temperate plants have an underdeveloped embryo and the embryo has to grow before germination (Baskin and Baskin, 2014). The seed is classified as having morphological dormancy (MD) when an underdeveloped embryo grows and radicle emerges in about 30 days under suitable conditions (Nikolaeva, 1977; Baskin and Baskin, 2014). However, if radicle emergence is delayed and the seeds require cold (0 - 10°C) and/or warm (≥15°C) temperatures for embryo growth and germination, they have morphophysiological dormancy (MPD), MD and physiological dormancy (PD) combined. Additionally, someseeds have physical dormancy (PY) due to water-impermeable seeds or fruit coats. MPD is divided into nine levels of dormancy based on (1) temperature requirements for embryo growth and germination, and (2) effect of gibberellic acid (GA3) on the dormancy breaking (Table 1). Warm (usually 15°C or above) temperatures stimulate embryo growth in seeds with simple MPD, while seeds with complex MPD require cold (usually 0 - 10°C) temperatures for embryo growth. These two types of MPD are subdivided into three levels of dormancy, including non-deep, intermediate, and deep. Although GA3 increases germination in seeds with non-deep and intermediate MPD, seeds with deep MPD cannot germinate (Nikolaeva, 1977). Seed dormancy requiring warm temperatures for radicle emergence followed by cold temperatures for shoot emergence is generally known as “epicotyl dormancy” (Kondo et al., 2011; Baskin and Baskin, 2014).
Table 1. The nine levels of MPD (modified from Baskin and Baskin, 2004; Baskin and Baskin, 2014) based on temperature requirements to break seed dormancy and germination
y+, Yes; +/‑, yes/no; ‑, no.
xGA3 substitutes for warm but not for cold stratification.
This sophisticated dormancy classification system has been applied to species of North America’s eastern deciduous forest (Baskin and Baskin, 2014). Several studies have also been conducted for seeds with MPD in temperate forests in East Asia and Europe (Vandelook and Van Assche, 2009; Kondo et al., 2011; Kırmızı et al., 2018). Seeds of spring ephemeral plants native to Korea have an underdeveloped embryo (Lee et al., 2012). For example, seeds of Aquilegia buergeriana and Pulsatilla tonkangensis have MD, whereas seeds of Leontice microrhyncha (synonym of G. microrrhynchum), Jeffersonia dubia, Adonis amurensis, Ranunculus crucilobus, Erythronium japonicum, and Trillium tschonoskii have MPD (Lee et al., 2012). For example, seeds of Heloniopsis koreana and H. tubiflora have both MD and non-deep simple MPD (Lee et al., 2014); some proportion of the seeds with MD germinated within 30 days, while the rest germinated by GA3 and light. Seeds of J. dubia have deep simple MPD where seeds germinated after warm followed by cold stratification (Rhie et al., 2015). Seeds of Cicuta virosa have deep complex MPD that require cold stratification to germinate (Cho et al., 2018).
G. microrrhynchum belongs to the family Berberidaceae and is a perennial herb in forests in Korea and Northeast China (Lee, 1980). Their distribution is restricted to six localities (Gariwangsan, Geumdaebong, Jabyongsan, Jeombongsan, Odaesan, and Taebaeksan) in South Korea and nine localities in the northern part of Korea (Im, 1996). The plant produces approximately 4-cm-long cymes bearing bright-yellow hermaphrodite flowers that bloom in early spring. Berries of 8 mm in diameter are produced in each pollinated flower. While some believe this plant spreads its seeds by animals or birds, no dispersal vector can be found in the field (Chang et al., 2004). Rather, seeds are dispersed by gravity. Thus, the wild populations are overcrowded in small areas. This taxon may become extinct in the wild because its occurrence and occupancy are reduced (Chang et al., 2004). Red list criteria categorize G. microrrhynchum as vulnerable in Korea (Chang et al., 2005). Although information about propagation is critical, vegetative propagation of this plant is difficult because the rhizome barely develops and the root tuber is 30 cm in depth in the soil (KBIS, 2014). Therefore, seed propagation is almost the only way to propagate this species.
Lee et al. (2012) observed that G. microrrhynchum have underdeveloped embryos that occupy approximately 7% of the seed length, indicating the presence of MD. They also tested seed germination in the growth chamber (under 25/15°C) but failed to germinate within 30 days, so they concluded that seed of G. microrrhynchum had MPD. However, they did not find the requirement to promote germination in both natural or artificially controlled environments as well as the kind of seed dormancy of this species. While KBIS (2014) reported that G. microrrhynchum seeds germinate in early spring, there is no information available for its seed dormancy and germination ecology. As additional information on conditions required for germination of this species is needed for propagation and conservation, the purpose of this experiment was to describe the dormancy type present in G. microrrhynchum seeds.
Materials and Methods
Preparation of Seeds
Mature seeds were collected from a natural population of G. microrrhynchum in Gangneung (37°54'15"N, 128°78'59"E), Korea, on May 23, 2012 and May 28, 2013. Seeds were placed at room temperature (20 - 25°C) for 2 days. Dried seeds were kept in plastic bags at 5°C until used. We conducted experiments within 2 weeks of collection.
Imbibition
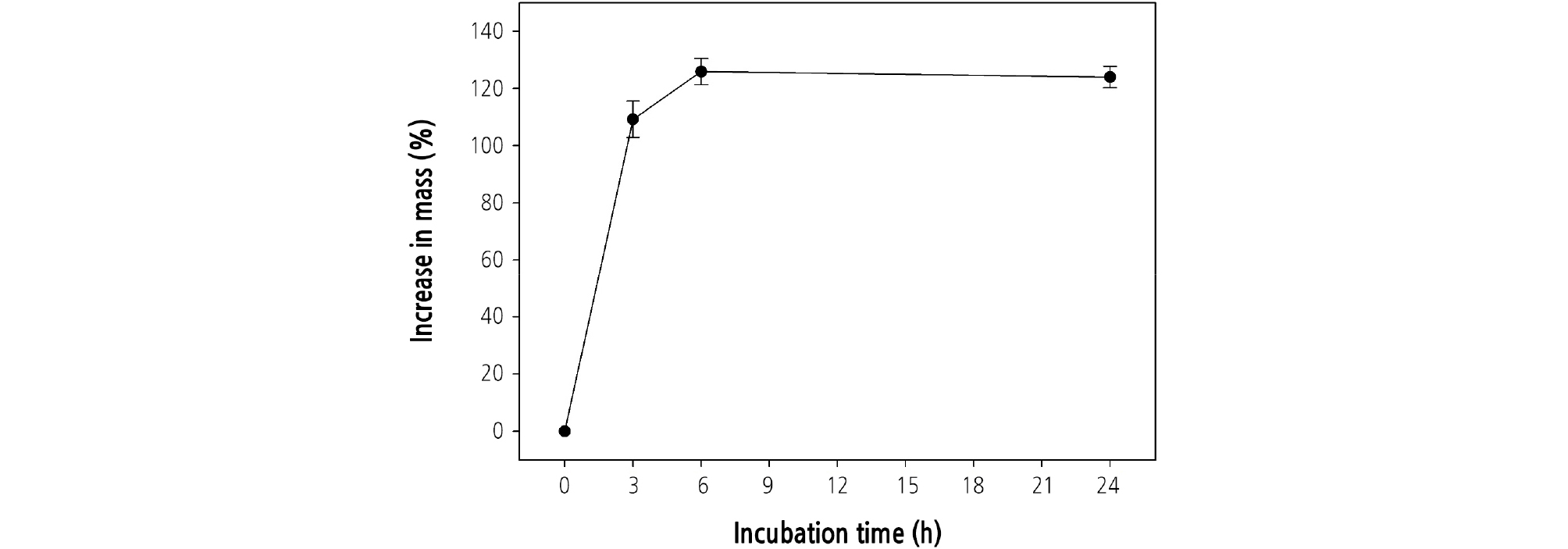
To investigate whether seeds have PY, the water uptake rate was checked in three replicates of five seeds. Seeds were placed on two sheets of Whatman #1 filter paper wetted with distilled water in plastic Petri dishes (90-mm diameter) at room temperature. Seeds were weighed after 0, 3, 6, or 24 h of incubation. Percent water uptake was calculated by % Ws = [(Wi ‒ Wd)/Wd] × 100, where Ws = the increase in mass of seed, Wi = the seed mass after a given interval of imbibition, and Wd = the initial seed mass at 0 h.
Embryo Growth, Radicle Emergence, and Shoot Emergence in the Field
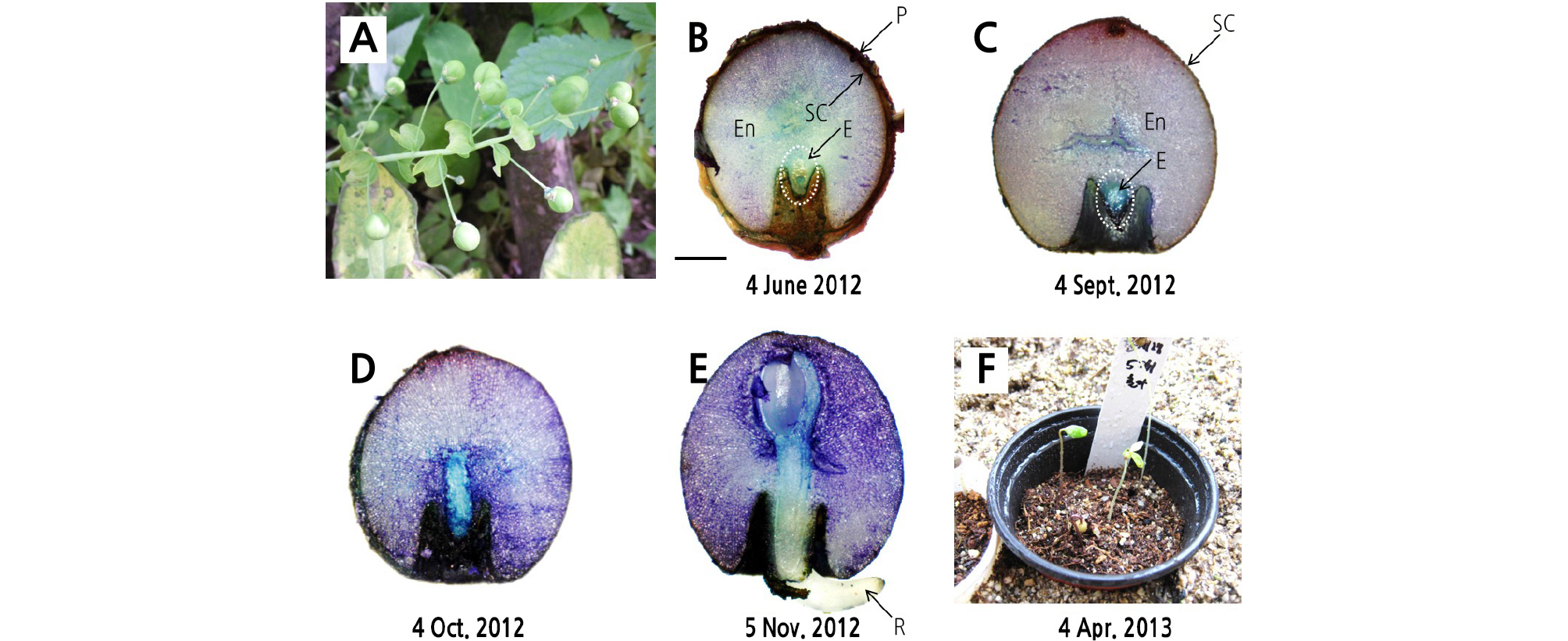
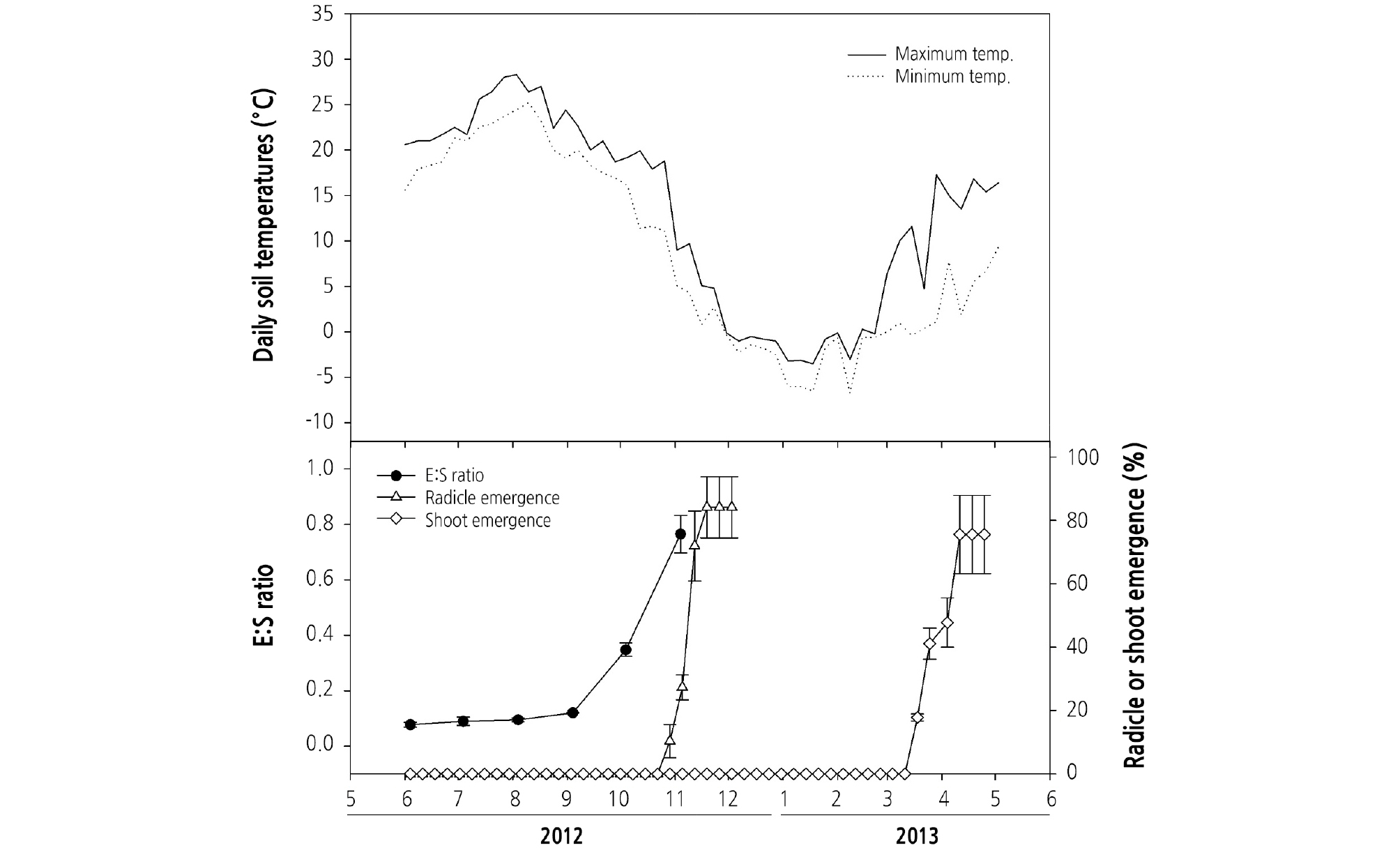
To monitor the embryo growth and radicle emergence under natural conditions, seeds were mixed with sand in a 1:1 volume ratio in polyester mesh bags and were placed in soil approximately 3 cm deep in an experimental garden at the Seoul National University, Seoul, Korea in June 2012. To measure soil temperatures, daily maximum and minimum temperatures at a soil depth of 3 cm were determined with a temperature data logger (Watch Dog Model 450; Spectrum Technologies, Inc., Plainfield, IL, USA) (Fig. 3). On the fourth day of every month from June 4, 2012 to November 4, 2012, we exhumed five seeds randomly from bags. Seeds were cut in half using a razor blade, and the embryo growth was observed with an ocular micrometer. Seeds were stained with 0.05% toluidine blue to observe the embryo. The seed length was expressed by the ratio of embryo length to seed length (E:S ratio).
In June 2012, three replicates of 30 seeds in a fine mesh polyester bag were sown at a soil depth of 3 cm in the garden. Bags were exhumed and radicle emergence was checked weekly. Seeds with a 2-mm-long radicle were counted and removed. We also observed the seedling emergence from seeds buried under natural conditions. Three replicates of 20 seeds were sown at a depth of 1 cm in plastic pots filled with horticultural substrate (Baroker, Seoul Bio, Seoul, Korea). These pots were covered with a net and buried in a shady place in the garden. Emerged seedlings were counted every week.
Effect of Temperature on Radicle Emergence
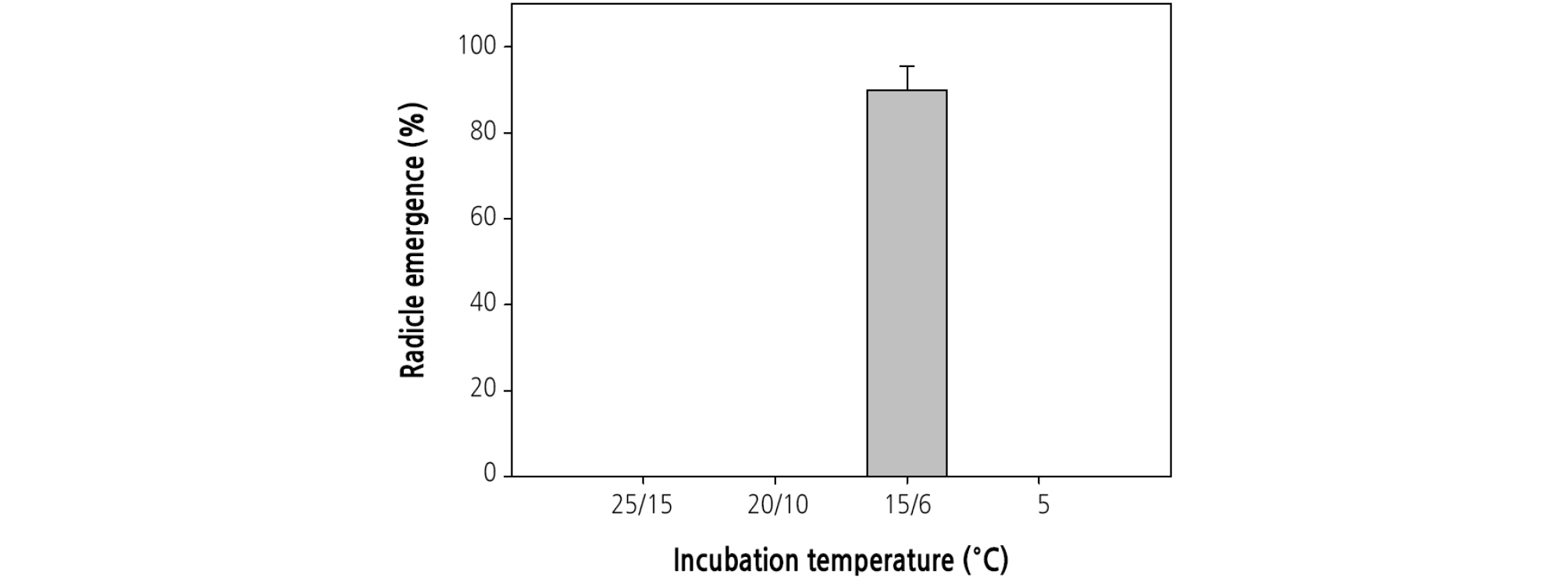
To determine the optimum temperatures for radicle emergence, intact seeds were incubated at 5°C, 25/15°C, 20/10°C, and 15/6°C (12/12 h) in the growth chamber (DS-13MCLP, Dasol Scientific, Hwaseong, Korea) from June 6, 2012 to October 24, 2012. In all of the temperature regimes, 12-h light was provided by cool white fluorescent lamps (photosynthetic photon flux of 30 - 40 µmol·m-2·s-1 in a light period). Three replicates of 20 seeds were incubated on sand moistened with distilled water in plastic Petri dishes (9.0-cm diameter) that were sealed with parafilm to prevent drying. The final germination was measured after 20 weeks of seed incubation. For the ecophysiological studies, many experiments used 5°C to simulate winter temperature, 25/15°C for summer, 20/10°C for early autumn and for late spring, and 15/6°C for late autumn and for early spring (Kondo et al., 2011; Baskin and Baskin, 2014). At first, these temperatures were chosen for Kentucky and adjacent areas in the United States. The temperature of Korea could have been used, but the temperatures were not very different and so we used those mentioned above.
Cold Temperature Requirements for Shoot Emergence
Seeds with epicotyl dormancy require dormancy breaking treatments for the hypocotyl or plumule to grow after radicle emergence (Baskin and Baskin, 2014). On June 8, 2012, two polyester bags of 30 seeds each were sown at a depth of 3 cm in the experiment garden until radicle emergence. On November 20, 2012, radicle-emerged seeds were exhumed from the soil. One part was incubated to room temperature (20 - 25°C), the other was incubated to 5°C, and both were checked for shoot emergence after 12 weeks.
Effect of GA3 Treatment on Radicle Emergence
Three replicates of 20 seeds were soaked in GA3 solutions with different levels of concentration (0 or 10, 100, and 1,000 mg·L-1) for 24 h at room temperature on June 12, 2013. Then, seeds were incubated at 20/10°C. Radicle or shoot emergence was monitored weekly to determine the ability of GA3 to break dormancy (Baskin and Baskin, 2014).
Results
Imbibition
Seed weight increased to 109.3% ± 6.4% (mean ± SE) after 3 h of imbibition and to 125.9% ± 4.6% after 6 h (Fig. 1). Seed mass did not increase from 6 h after the onset of imbibition.
Embryo Growth, Radicle Emergence, and Shoot Emergence in the Field
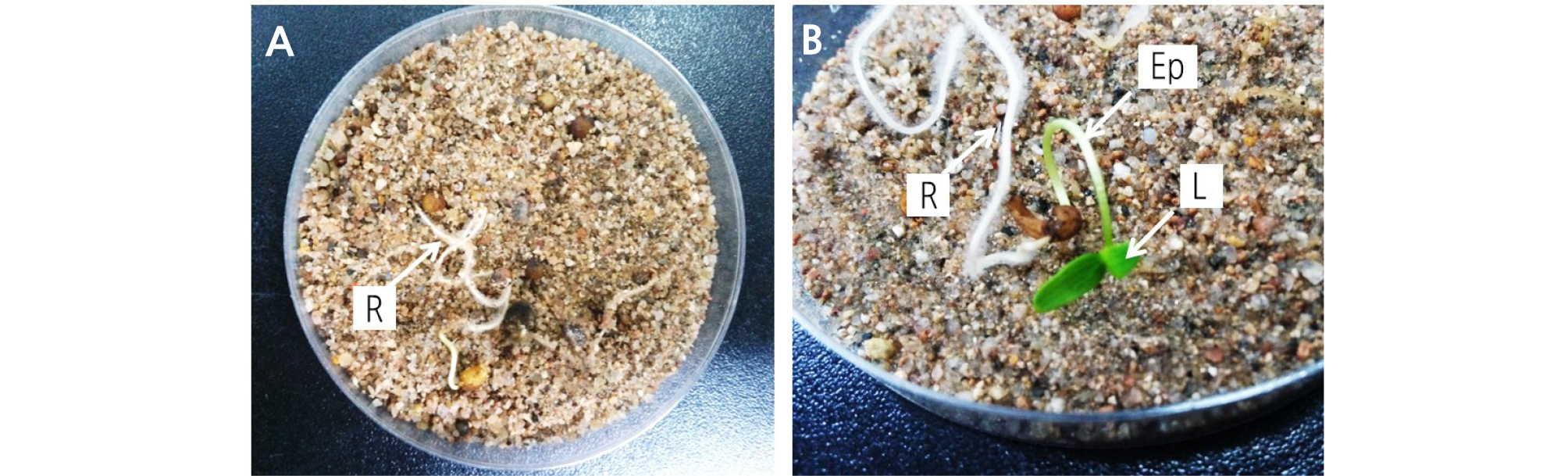
G. microrrhynchum seeds were surrounded by bright-green pericarp at harvest time (Fig. 2A), but this pericarp removed itself when sown under the soil (Fig. 2B and 2C). The initial embryo length at harvest was 0.28 ± 0.05 mm, where the E:S ratio was 0.08 (Figs. 2B and 3). Embryos did not grow between June and September when the soil temperature range was 15 - 28°C (Figs. 2C and 3). However, embryo length increased rapidly in October and E:S ratio reached 0.8 in November (Figs. 2D and 3). During the experiment, the maximum and minimum soil temperatures were approximately 19°C and 4°C, respectively. Radicle emergence occurred between late October and November, with more than 80% of seeds germinated by 19 November 19, 2012 (Figs. 2E and 3). Although most of the seeds germinated in November, shoot emergence did not occur until the following spring (Fig. 3). Shoot emergence began on March 18, 2013 and increased to 75.6% ± 12.4% by April 11, 2013 (Figs. 2F and 3).
Effect of Temperature Regimes on Radicle Emergence
Incubation at 15/6°C promoted radicle emergence (86.7% ± 8.8%, 20 weeks after incubation) (Fig. 4). However, at 25/15°C, 20/10°C, or 5°C, radicle emergence did not occur after 20 weeks.
Effect of Cold Temperature on Shoot Emergence
After radicle emergence, seeds incubated at room temperature did not produce the shoot (Fig. 5A). However, the shoot emergence occurred from seeds incubated at 5°C
Effect of GA3 Treatment on Radicle Emergence
No seeds germinated under various concentrations of GA3. In addition, there was no change in embryo length according to GA3 treatments (data not shown).
Discussion
It is known that G. microrrhynchum seeds germinate in spring (KBIS, 2014). However, we found that root emergence occurred first in autumn and then shoot emergence began in the following spring in this species (Fig. 2). Seeds having epicotyl dormancy show the delay in shoot emergence after root emergence (Barton, 1933). Among native species of Korea, seeds of G. microrrhynchum (this study) and Polygonatum odoratum (Kang et al., 1996; Kang et al., 1999) have been shown to have epicotyl dormancy. The epicotyl dormancy is considered an adaptive mechanism for plants to their environment; the above parts of plants (i.e., cotyledons and shoots) are not exposed to predators and are protected from chilling injury during winter, while there is an additional effect of advancing the root before shoot emergence (Baskin and Baskin, 1985). This well-developed root system can then provide a better supply of water and nutrients to the developing shoot at the beginning of spring.
The observed increase in seed mass proves that the seed coat of G. microrrhynchum is permeable to water (Fig. 1). Thus, seeds of this taxon do not have PY. The seeds of G. microrrhynchum have MPD because they had an underdeveloped embryo and failed to germinate within 30 d (Fig. 2; Lee et al., 2012). An underdeveloped embryo began to grow in early autumn after they had undergone a summer season, not winter (Fig. 3). This is a typical pattern of simple MPD types. G. microrrhynchum seeds also had epicotyl dormancy because there was a delay in shoot emergence after radicles had emerged. There are two levels of epicotyl dormancy, depending on whether cold stratification is required (deep simple epicotyl MPD) or unable to break the dormancy (non-deep simple epicotyl MPD) (Baskin and Baskin, 2014). Following cold stratification at 5°C, G. microrrhynchum seeds produced shoots (Fig. 5). Moreover, GA3 did not promote embryo growth and radicle emergence in this species. In summary, we conclude that G. microrrhynchum seeds have deep simple epicotyl MPD.
Deep simple epicotyl MPD has been found in seeds of at least 10 families, including Ranunculaceae, Liliaceae, Aliaceae, Paeoniaceae, Amaryllidaceae, Caprifoliaceae, Boraginaceae, Aristolochiaceae, Melanthiaceae, and Berberidacea (Baskin and Baskin, 2014). In Beberidaceae, Podophyllum hexandrum was the only species known to have deep simple epicotyl MPD until now (Kushwaha et al., 2008). In this study, we found that G. microrrhynchum also has the same dormancy type in the family Beberidaceae. Jeffersonia diphylla and J. dubia also belong to this family and have deep simple MPD with no epicotyl dormancy (Baskin and Baskin, 1989; Rhie et al., 2015). High or warm temperatures are required for embryo growth in seeds with deep simple MPD and deep simple epicotyl MPD. In seeds with deep simple epicotyl MPD, the root emerges spontaneously after completion of embryo growth and then cold stratification is needed for shoot emergence. However, seeds with deep simple MPD have deep dormancy in both root and shoot and require warm followed by cold stratification for germination. Because seeds in the same genera often have a similar type of seed dormancy, knowing the germination phenology of some species will enable better design of propagation protocols to revive wild populations for other species in the same genus. In the 15 genera of the Berberidaceae family, there have been studies classifying seed dormancy in just three genera (Jeffersonia, Podophyllum, and Gymnospermium), such that further research is needed for other genera.
Although the time and temperature when embryo growth and radicle emergence varies among species having epicotyl dormancy, it generally requires temperatures that correspond to autumn in temperate regions. The radicle of Asarum canadensis (Baskin and Baskin, 1986) and Allium tricoccum (Baskin and Baskin, 2014) seeds emerged in autumn when the temperature ranged from 21.2°C to 11.1°C in Kentucky, USA, and those of Cimicifuga racemosa and Hepatica acutiloba (Baskin and Baskin, 1985) germinated in lower temperatures ranging from 13.7°C to 5.6°C. In Narcissus hispanicus, the radicle emergence occurred in October when daily average maximum and minimum temperatures were 19.4°C and 7°C, respectively (Copete et al., 2011). Epicotyl dormancy can be broken by exposing Paeonia suffruticosa seeds to temperatures ranging from 1°C to 10°C, with 5°C optimal (Barton, 1933). In this study, the embryos of G. microrrhynchum grew rapidly and radicles emerged in October when the mean maximum and minimum temperatures were about 19°C and 4°C, respectively (Fig. 3), while the optimum laboratory temperatures for radicle emergence were 15/6°C (Fig. 4). As with other plants with epicotyl dormancy, the temperature for radicle emergence of G. microrrhynchum ranges from 5°C to 20°C.
In summary, seeds of G. microrrhynchum had an underdeveloped embryo and failed to germinate even in favorable conditions for germination. An underdeveloped embryo had to grow before the radicle could emerge, which required high or warm temperatures. Radicles emerged in autumn after embryo growth had been completed. Further, the shoot only emerged after seeds were subjected to cold temperatures. Under laboratory conditions, radicles emerged at 15/6°C within 20 weeks, and cold stratification (5°C) broke epicotyl dormancy to produce the shoot. This research contributes to the germination ecology knowledge of G. microrrhynchum and provides information on how to germinate other dormant seeds for propagation.