Introduction
Materials and Methods
Plant Materials and Treatments
Assessments of Fruit Quality Attributes
Statistical Analyses
Results
Discussion
Introduction
In addition to sweet persimmon fruit, such as the cultivar ‘Fuyu’ (Itamura et al., 2005), astringent persimmon (Diospyros kaki Thunb.) fruit are also widely used in the production of processed persimmon commodities. While sweet persimmon fruit can be consumed fresh, astringent persimmon fruit cannot be consumed directly due to their bitter flavor. Thus, the astringent persimmon cultivars, such as ‘Tonewase’, ‘Rojo Brillante’, ‘Sangjudungsi’, and ‘Triumph’, are generally used in the production of a wide range of processed products, such as dried persimmon, semi-dried persimmon, ripe persimmon, ice-soft persimmon, and spreadable products (Yamada et al., 2009; Castelló et al., 2011; Li, 2013; Choi et al., 2017; Chung et al., 2017). After harvest, it is strongly recommended that the astringent persimmon fruit are stored at low temperature to preserve freshness (Wei et al., 2015; Yoo et al., 2016). During cold storage, astringent persimmon fruit ripen faster, but also develop chilling injury such as peel blackening and flesh browning (Grant et al., 1992; Collins and Tisdell, 1995; Salvador et al., 2005).
The astringent persimmon fruit should be processed to remove astringency by employing several commercial techniques, including high levels of CO2 gas, warm water, and ethanol vapor treatments (Khademi et al., 2010; Novillo et al., 2014; Chung et al., 2015; Chung et al., 2017; Min et al., 2018). These deastringency techniques reduce the soluble tannin content (Khademi et al., 2010; Novillo et al., 2014; Chung et al., 2015; Min et al., 2018). Among these techniques, the application of high levels of CO2 gas is widely employed to retain fruit quality during cold storage without causing physiological damage (Khademi et al., 2010). However, this deastringency treatment might contribute to an additional reduction in fruit firmness before (Novillo et al., 2013; Khademi et al., 2014; Wang et al., 2017) and after cold storage, and during shelf life (Khademi et al., 2014). The reduction in flesh firmness is highly associated with heightened activity of pectin methylesterase (Khademi et al., 2014), indicating that deastringency treatments might be strongly associated with fruit softening during and after cold storage, but the relationship between deastringency treatments and fruit softening is cultivar dependent (Wang et al., 2017).
It is important to retain the firmness of astringent persimmon fruit to maintain a steady and reliable supply of fresh fruit for processing. Since its introduction into the tree fruit industry, the 1-methylcyclopropene (1-MCP) treatment technique has been widely employed for retaining the firmness of persimmon fruit during cold storage and throughout its shelf life (Tsviling et al., 2003; Salvador et al., 2004b; Win et al., 2018). Furthermore, fruit softening during cold storage can be delayed by 1-MCP treatment (Win et al., 2018). The maintenance of fruit firmness and the delay of fruit softening induced by 1-MCP treatment might be associated with the alleviation of chilling injury in cold-stored ‘Rojo Brillante’ persimmon fruit (Salvador et al., 2004a; Pérez-Munuera et al., 2009; Novillo et al., 2015). In addition, the fruit decay rate is highly reduced by 1-MCP treatment in astringent persimmon cultivars during cold and controlled atmosphere storage (Besada et al., 2014).
The combined application of deastringency and 1-MCP treatments is believed to retain fruit quality by lowering the soluble tannin content and by retaining fruit firmness or by delaying fruit softening during cold storage and throughout its shelf life. Numerous applications of this combination treatment, comprising high levels of CO2 gas and 1-MCP treatments, have been evaluated in ‘Rojo Brillante’ (Pérez-Munuera et al., 2009; Khademi et al., 2014) and ‘Niuxin’ (Min et al., 2018) cultivars during storage. Thus, it is important to elucidate the effects of a combination of deastringency and 1-MCP treatments on fruit quality attributes and the incidence of fruit physiological disorders in cold-stored astringent persimmon fruit. In this study, we aimed to test the hypothesis that the combined application of deastringency and 1-MCP treatments would be highly effective in retaining fruit quality and controlling the incidence of physiological disorders during cold storage. Therefore, the objectives of this study were to evaluate (1) the effects of a combination of these two treatments on the quality attributes and storage disorders of fruit of two different astringent persimmon cultivars during cold storage and (2) how long fruit storability can be extended by these treatments during cold storage.
Materials and Methods
Plant Materials and Treatments
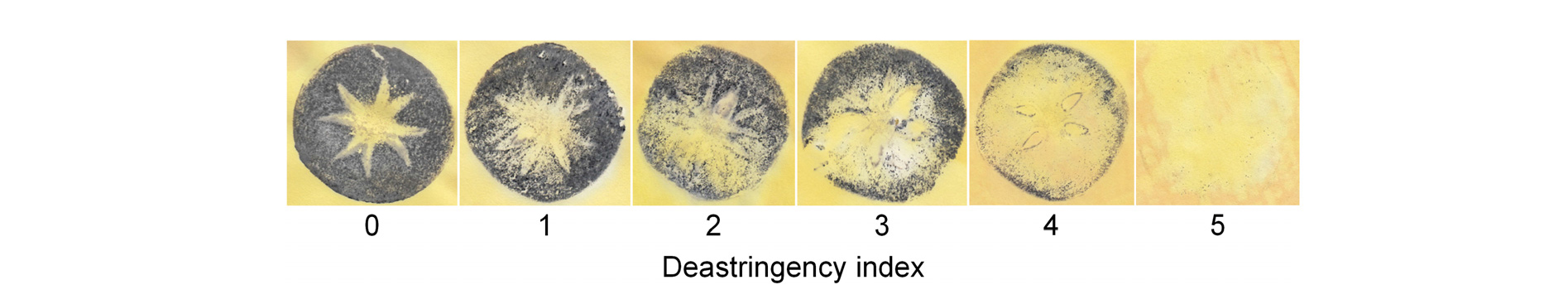
‘Tonewase’ and ‘Sangjudungsi’ persimmon (Diospyros kaki Thunb.) fruit were harvested from a commercial orchard in Sangju, the Republic of Korea. In 2017, ‘Tonewase’ fruit were collected on September 24 and ‘Sangjudungsi’ fruit were collected on October 13. The fruit were transported immediately to the Laboratory of Horticultural Crop Quality Management at Kyungpook National University in Daegu, the Republic of Korea. Persimmon fruit without visible deformities, injuries, or disease were selected. The treatments were as follows: (1) untreated control, (2) fumigation with 1 µL·L-1 1-MCP (SmartFreshSM, AgroFresh Co., Seoul, Korea) for 18 h in a sealed container, (3) deastringency treatment of ‘Tonewase’ and ‘Sangjudungsi’ fruit treated with 90% CO2 gas for 3 d and 7 d, respectively, in a sealed plastic container, (4) deastringency treatment followed by 1-MCP, and (5) 1-MCP followed by deastringency treatment. All the treatments were carried out at ambient temperature. Subsequently, all fruit were stored at -1°C with 90% relative humidity for up to 4 months. The efficacy of the deastringency treatment was confirmed by the blue print (FeCl3) approach (Eaks, 1967) as shown in Fig. 1. The deastringency index was subjectively assigned on a 0 to 5 scale based on the absence of soluble tannins in horizontal cross sections of persimmon fruit treated with 90% CO2 gas, where 0: 0% deastringency; 1: 20% deastringency; 2: 40% deastringency; 3: 60% deastringency; 4: 80% deastringency; and 5: 100% deastringency (Guan et al., 2017; Munera et al., 2017).
Assessments of Fruit Quality Attributes
The fruit quality attributes were assessed at monthly intervals for 4 months. The fruit quality attributes evaluated were: fresh weight loss, flesh firmness, soluble solids content (SSC), peel color variables [L*(lightness; 0-100), a*(redness), and b*(yellowness)] at the calyx-end and equator regions of the fruit, and incidence of physiological disorders. Fruit fresh weight (n = 9) was measured with an analytical balance using the same fruit at harvest and 1 d after cold storage. There was difference in the fresh weight of fruit between these two time points. Flesh firmness was determined using a digital penetrometer (Fruit Pressure Tester, FT-327, Alfonsine, Italy). Three measurements were obtained around the equator of each fruit (n = 9) and the results are expressed in newton (N) unit. Expressed juice from the penetrometer readings was used for SSC measurement with a refractometer (Model PR-201α; Atago Co., Ltd., Tokyo, Japan).
To measure ethylene production and respiration rate, three fruit were incubated in 1.6 L containers for 2 h. Then, 1 mL of the headspace gas sample was withdrawn from the containers. The sample was injected into a gas chromatograph (GC2010; Shimadzu Co., Tokyo, Japan) equipped with a flame ionization detector (FID) and fitted with a Porapak Q column (80/100 mesh, 1 m long; Young In Frontier Co., Seoul, Korea). Analyses were run isothermally with an oven temperature of 90°C, injector temperature of 100°C, and detector temperature of 200°C. The flow rate of helium was 25 mL·min-1. Ethylene was quantified by peak area using an external standard for calibration. Carbon dioxide was injected into a gas chromatograph equipped with a thermal conductivity detector (TCD) and fitted with a Porapak Q column (80/100 mesh, 1 m long; Young In Frontier Co., Seoul, Korea). The injector and detector temperatures were 100°C and the oven temperature was 90°C. The flow rate of helium was 15 mL·min-1. Carbon dioxide was quantified by peak area with an external standard used for calibration.
The rate and severity of peel blackening, fruit softening, decay, and wilting (n = 9) were subjectively evaluated throughout the storage period. The severity rate of peel blackening, fruit softening, decay, and wilting was scored as 0 = 0%, 1 = 1 - 10%, 2 = 11 - 25%, 3 = 26 - 50%, 4 = 51 - 75%, and 5 = 76 - 100% of total peel or flesh coverage (Lee et al., 2019).
Statistical Analyses
The data were subjected to analysis of variance (ANOVA) to determine the effects of main factors and interactions (Version 9.3; SAS Institute Inc., Carry, NC, USA). The least significant difference (LSD) test was applied for analysis of mean difference at p ≤ 0.05.
Results
Fruit fresh weight loss gradually increased during cold storage (p < 0.0001). However, the response pattern of fresh weight loss was highly different between cultivars, depending on the deastringency and 1-MCP treatments (p < 0.001). However, the deastringency treatment might be strongly associated with fresh weight loss in ‘Sangjudungsi’ persimmon fruit compared with that in control fruit. Furthermore, with respect to the timing of the deastringency treatment, deastringency after 1-MCP treatment resulted in relatively higher fresh weight loss than that with 1-MCP treatment after deastringency or deastringency treatment alone. This difference was apparent at the start of cold storage, based on deastringency. The duration of the deastringency treatment in ‘Sangjudungsi’ fruit was longer than that in ‘Tonewase’ fruit. Therefore, the difference in fresh weight loss was significantly higher in ‘Sangjudungsi’ fruit than in ‘Tonewase’ fruit (p < 0.0001, Fig. 2).
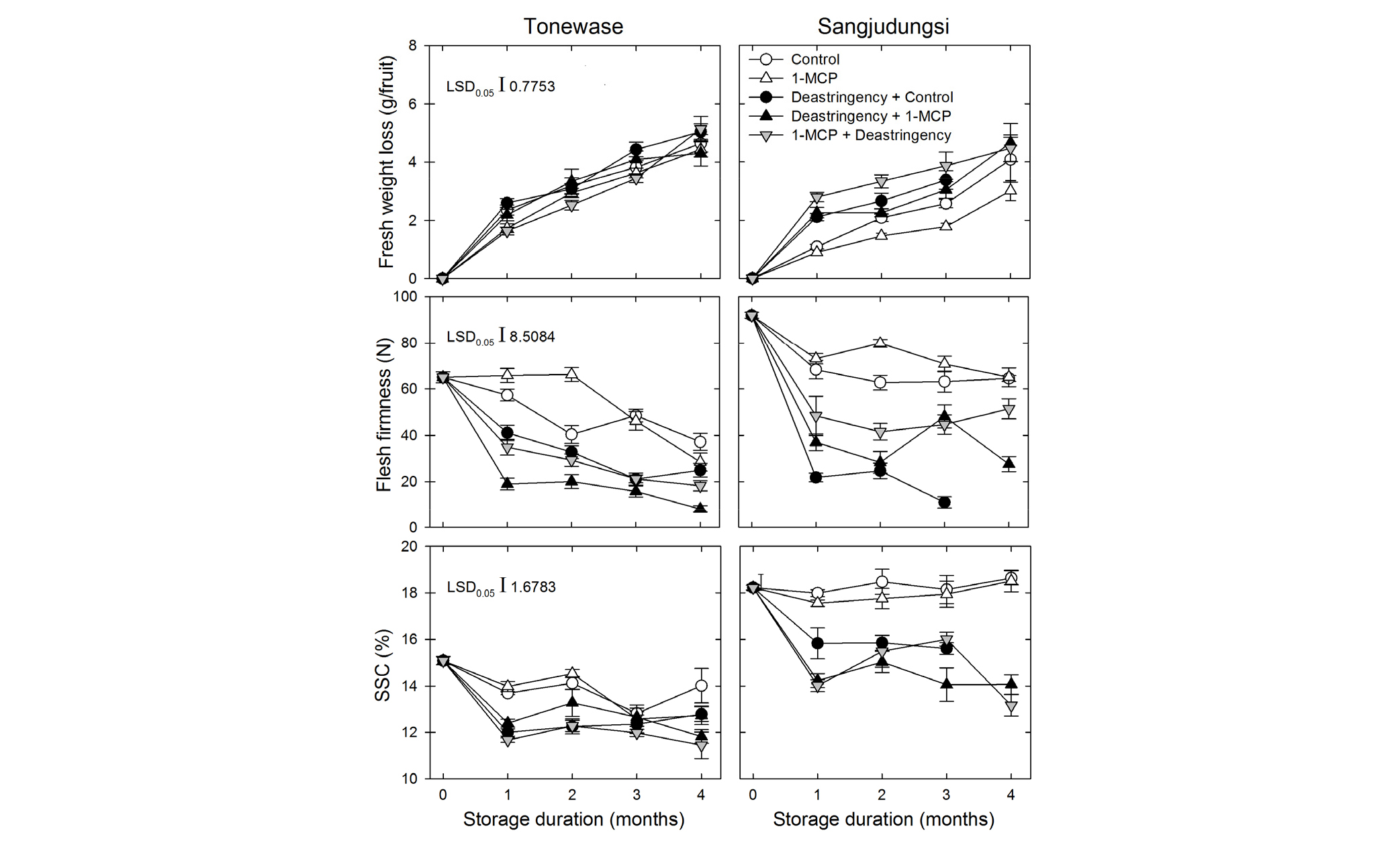
Fig. 2.
Fresh weight loss, flesh firmness, and soluble solids content (SSC) of ‘Tonewase’ and ‘Sangjudungsi’ fruit subjected to 1 µL·L-1 1-methylcyclopropene (1-MCP) treatment before or after the deastringency treatment with 90% CO2 for 3 d and 7 d, respectively, at ambient temperature during harvest, and then stored at -1°C for up to 4 months.
Fruit flesh firmness was highly reduced by the deastringency treatment compared with that by the 1-MCP treatment, regardless of the cultivar (p < 0.0001). Nevertheless, irrespective of the deastringency treatment, flesh firmness was higher with 1-MCP treatment than that of control fruit during cold storage. In general, the deastringency treatment reduced flesh firmness compared with the 1-MCP treatment alone, regardless of the timing of deastringency. Similar to its effect on fresh weight loss, the deastringency treatment reduced flesh firmness at the start of cold storage. Furthermore, the reduction in flesh firmness was considerably higher in ‘Sangjudungsi’ fruit than in ‘Tonewase’ fruit (Fig. 2).
The deastringency treatment reduced the SSC compared with that of the control and 1-MCP treatment alone (p < 0.0001). The SSC response was significantly different between the two cultivars (p < 0.0001). The SSC decreased marginally in ‘Tonewase’ during cold storage and remained unchanged in ‘Sangjudungsi’ only in the control and 1-MCP treatment (Fig. 2).
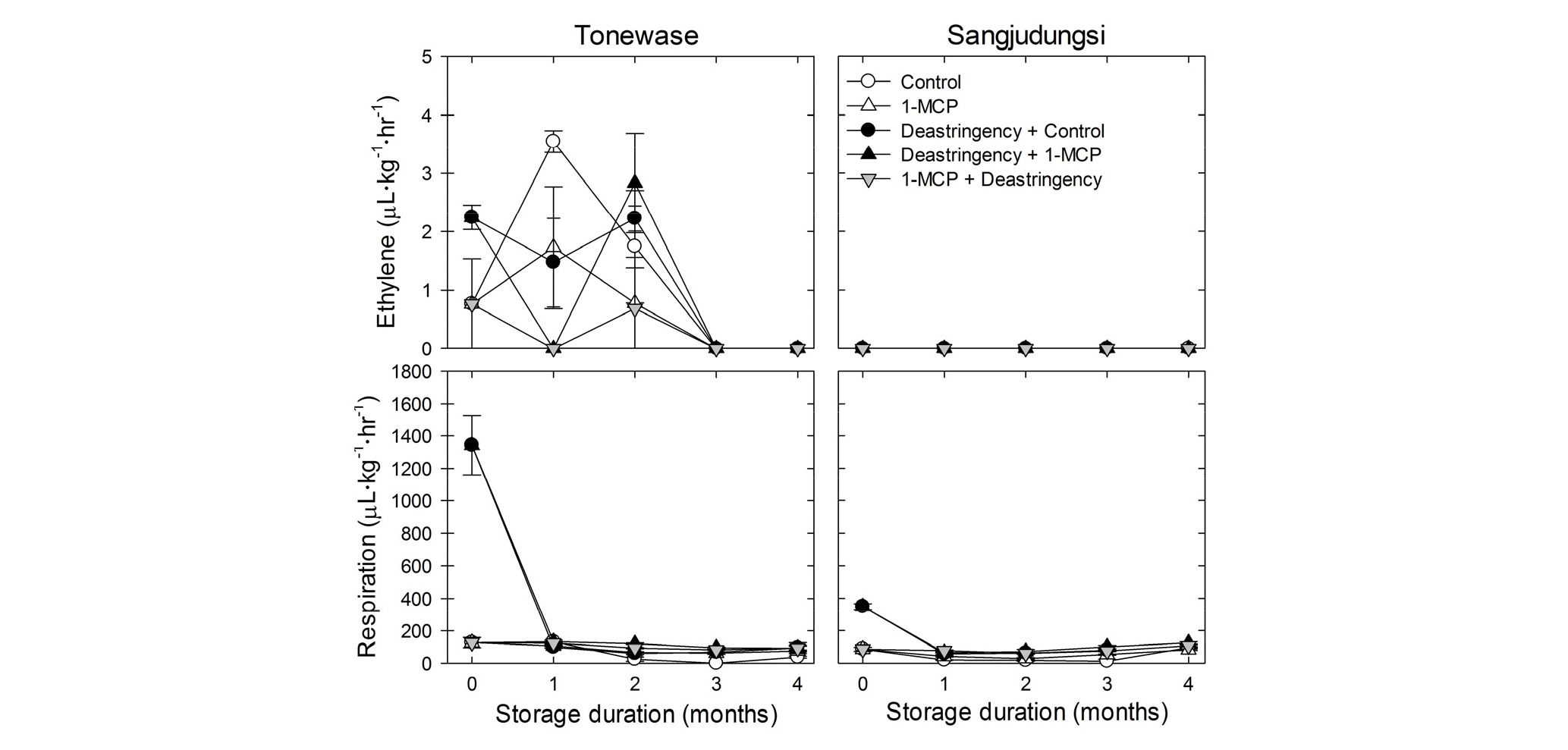
Ethylene production was not affected by deastringency or 1-MCP treatment in ‘Tonewase’ and ‘Sangjudungsi’ fruit during cold storage (Fig. 3). Furthermore, the respiration rate of fruit was only enhanced by the deastringency treatment right before cold storage. The respiration rate was higher in ‘Tonewase’ than in ‘Sangjudungsi’ during cold storage (Fig. 3).
The fruit peel color variables, lightness (L*), redness (a*), and yellowness (b*), gradually decreased during cold storage (p < 0.0001). The reduction in these color variables at the calyx-end and equator peel regions was significa Fig. 2. Fresh weight loss, flesh firmness, and soluble solids content (SSC) of ‘Tonewase’ and ‘Sangjudungsi’ fruit subjected to 1 µL·L-1 1-methylcyclopropene (1-MCP) treatment before or after the deastringency treatment with 90% CO2 for 3 d and 7 d, respectively, at ambient temperature during harvest, and then stored at -1°C for up to 4 months. ntly higher in ‘Sangjudungsi’ than in ‘Tonewase’ (p < 0.0001). Specifically, 1-MCP treatment after the deastringency treatment caused a higher reduction in peel color variables at both the calyx-end and equator regions of fruit of ‘Tonewase’ during cold storage than in those of ‘Sangjudungsi’. The difference in peel redness between the two peel regions was higher in ‘Sangjudungsi’ than in ‘Tonewase’ during cold storage (Fig. 4).
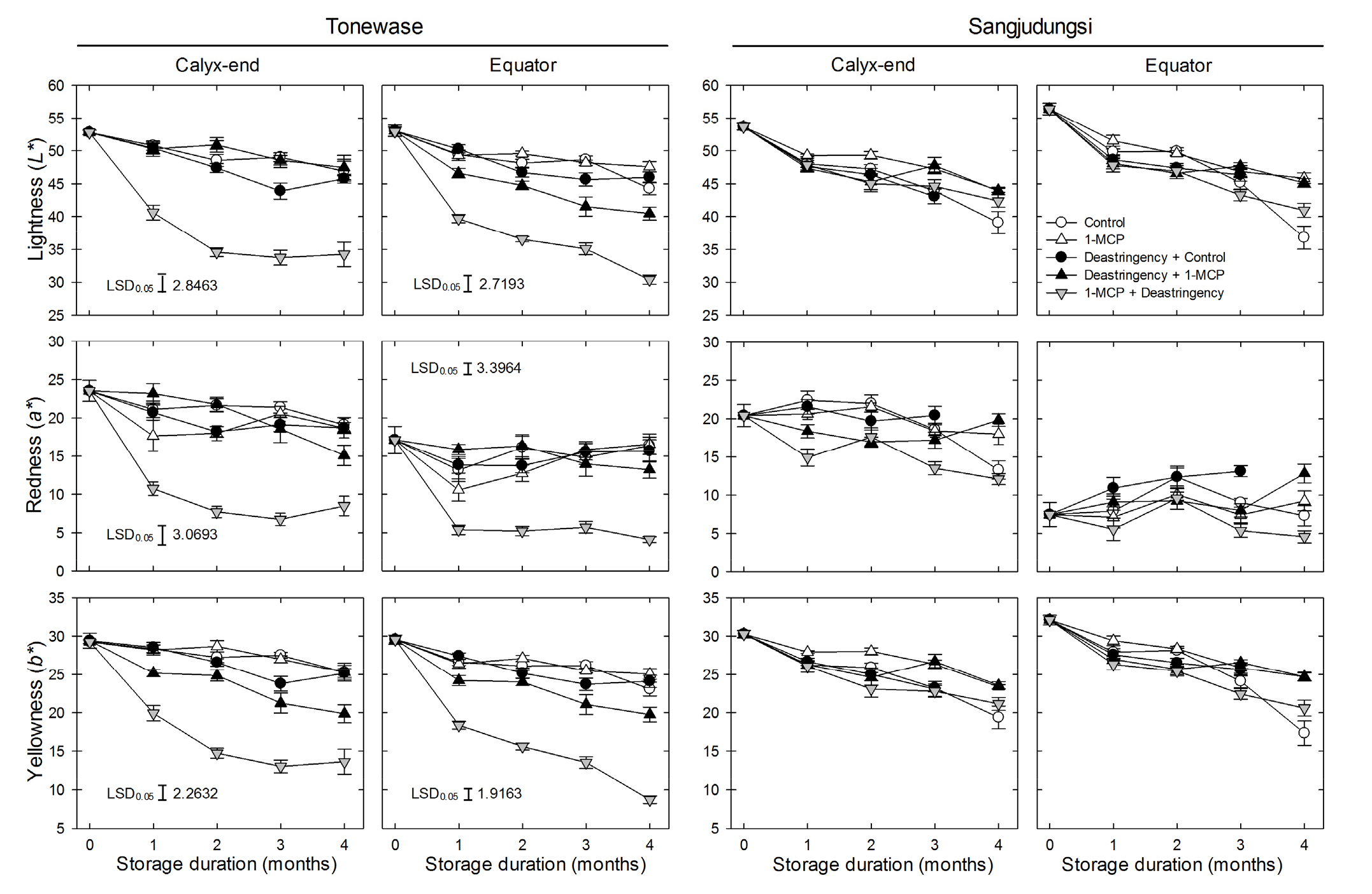
Fig. 4.
Peel color variables [lightness (L*), redness (a*), and yellowness (b*)] of ‘Tonewase’ and ‘Sangjudungsi’ fruit subjected to 1 µL·L-1 1-methylcyclopropene (1-MCP) treatment before or after the deastringency treatment with 90% CO2 for 3 d and 7 d, respectively, at ambient temperature during harvest, and then stored at -1°C for up to 4 months.
The peel blackening rate was significantly higher in ‘Tonewase’ than in ‘Sangjudungsi’ during cold storage (p < 0.001). Furthermore, the fruit softening rate was significantly higher in ‘Tonewase’ than in ‘Sangjudungsi’ (p < 0.0001). Interestingly, the combination of deastringency and 1-MCP treatments enhanced fruit softening in ‘Tonewase’ at the end of cold storage. However, this effect was not observed in ‘Sangjudungsi’. The fruit decay rate was significantly higher in ‘Tonewase’ than in ‘Sangjudungsi’ during cold storage (p < 0.0001). Furthermore, the fruit wilting rate was also significantly higher in ‘Tonewase’ than in ‘Sangjudungsi’ fruit during cold storage (p < 0.0001). Fruit wilting was highest in the combination of 1-MCP and deastringency treatments during cold storage (p < 0.0001). Similar to the fruit decay rate, the fruit wilting rate was not affected by 1-MCP treatment or by deastringency in ‘Sangjudungsi’ during cold storage (Fig. 5).
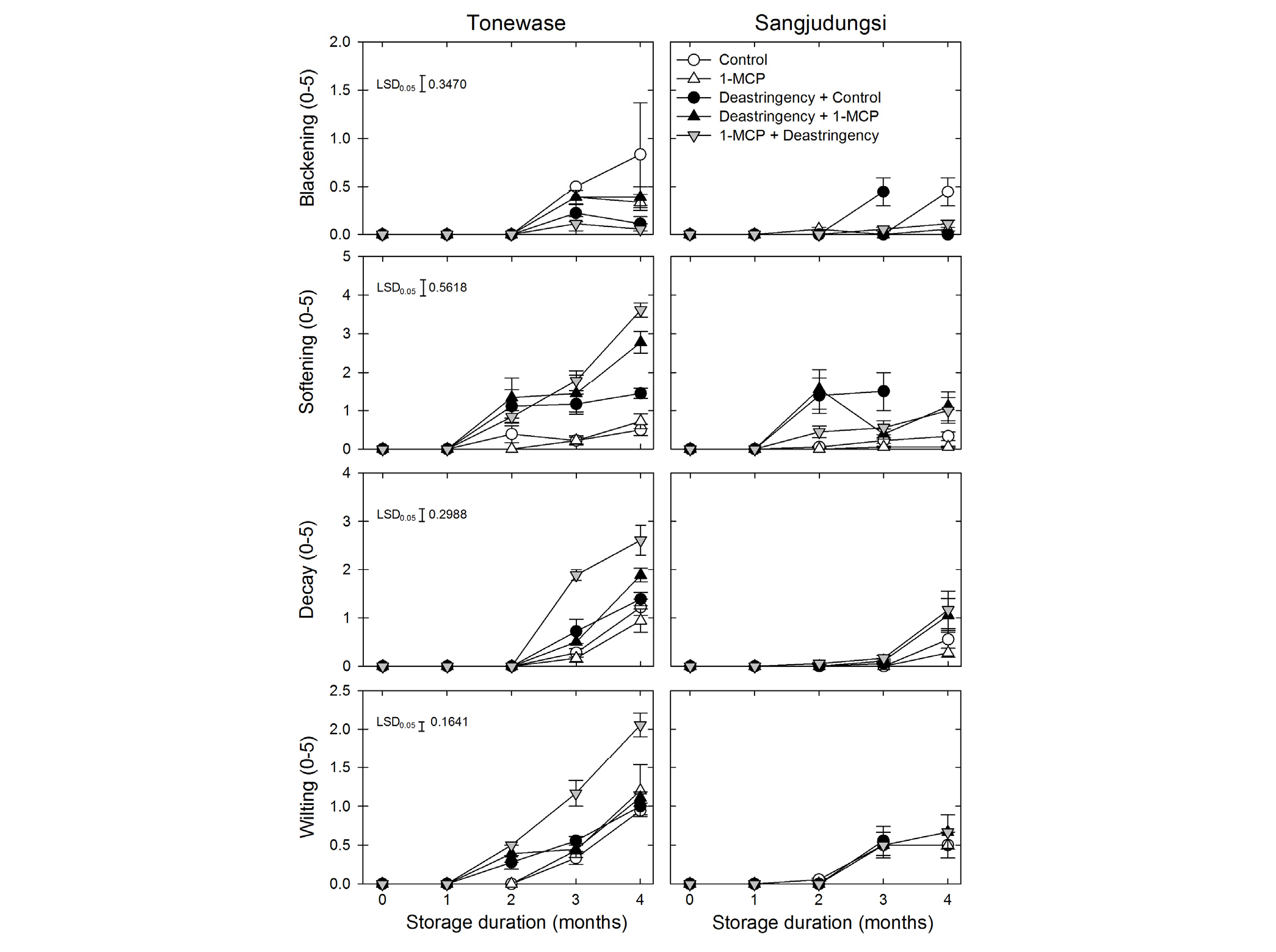
Fig. 5.
Physiological disorders and decay rate of ‘Tonewase’ and ‘Sangjudungsi’ fruit subjected to 1 µL·L-1 1-methylcyclopropene (1-MCP) treatment before or after the deastringency treatment with 90% CO2 for 3 d and 7 d, respectively, at ambient temperature during harvest, and then stored at -1°C for up to 4 months. The severity of peel blackening, fruit softening, decay, and wilting was scored as 0 = 0%, 1 = 1% - 10%, 2 = 11% - 25%, 3 = 26% - 50%, 4 = 51% - 75%, and 5 = 76% - 100% of total peel or flesh coverage.
Discussion
After harvest, astringent persimmon fruit are generally stored under cold conditions before their use in the production of processed persimmon products. However, the astringent taste would still persist (Kim et al., 2017). Thus, several commercial techniques for deastringency are used in the persimmon fruit industry (Kato, 1990; Oshida et al., 1996; Yamada et al., 2002; Chung et al., 2017; Zhu et al., 2018). These techniques are associated with reducing the soluble tannin content in persimmon fruit (Tanaka et al., 1994; Chung et al., 2015; Fang et al., 2016). In this study, a 90% CO2 gas treatment was applied for deastringency in both persimmon cultivars, although the response to high levels of CO2 gas differed by cultivar. Complete removal of fruit astringency by CO2 gas treatment required 3 d for ‘Tonewase’ and 7 d for ‘Sangjudungsi’. This difference in effectiveness of the CO2 gas treatment indicates different physiological responses of persimmon cultivars. As a consequence, these astringency removal techniques can reduce fruit firmness, thereby enhancing fruit softening (Pesis and Ben-Arie, 1984; Kato, 1990; Chung et al., 2015). As shown in Fig. 2, flesh firmness was significantly decreased by the deastringency treatments, irrespective of the cultivar. This might occur because deastringency techniques result in the up-regulation of certain fermentative metabolites, such as ethanol and acetaldehyde (Pesis and Ben-Arie, 1984; Fang et al., 2016). Thus, we assumed that the reduction in flesh firmness by deastringency treatments might be indirectly or directly associated with fresh weight loss during cold storage. Regardless of the timing of the 1-MCP treatment, the deastringency treatment enhanced fresh weight loss in ‘Sangjudungsi’, but the effect was relatively less in ‘Tonewase’. Furthermore, the deastringency treatments decreased the SSC during cold storage. In ‘Niuxin’ persimmon fruit, the combination treatment with CO2 and 1-MCP significantly delayed the reduction in SSC compared with that by CO2 treatment alone (Min et al., 2018).
Along with the deastringency treatment, the 1-MCP treatment was also applied to retain fruit quality during cold storage. Flesh firmness was retained during cold storage by the deastringency treatment, regardless of the cultivar, but fruit firmness was not affected by the 1-MCP treatment alone. Furthermore, the combination of the 1-MCP treatment with the deastringency treatment reduced the SSC and enhanced fruit softening and decay in ‘Tonewase’ compared with ‘Sangjudungsi’, irrespective of the timing of 1-MCP and deastringency. This indicates that the responsiveness of fruit to the combined treatment with 1-MCP and CO2 might be cultivar specific. However, the combined application of CO2 and 1-MCP treatments retained fruit firmness, SSC, and titratable acidity, when compared with those by deastringency treatment alone after 30 d of cold storage and 5 d after removal from cold storage in the persimmon cultivar ‘Rojo Brillante’ (Khademi et al., 2014). In ‘Niuxin’, the application timing of 1-MCP and deastringency treatments significantly affected soluble tannin content, firmness, and SSC during cold storage (Min et al., 2018). Thus, it was considered that the difference in responses to the combined application of 1-MCP and deastringency treatments is highly dependent on the cultivar.
Ethylene production and respiration were not affected by 1-MCP treatment. However, the deastrigency treatment caused an increase in the respiration rate right before cold storage, irrespective of whether or not the 1-MCP treatment was included. However, the 1-MCP followed by deastringency treatment did not enhance fruit respiration. Ethylene production and respiration rates were not affected by 1-MCP treatment in ‘Fuyu’ persimmon at ambient temperature (Choi, 2010), ‘Rendaiji’ persimmons (Ortiz et al., 2005), and cold-stored ‘Tonewase’ astringent persimmons (Win et al., 2018). Also, 1-MCP and deastringency treatments did not affect fruit respiration and ethylene production in cold-stored ‘Rojo Brillante’ persimmons (Khademi et al., 2014). A wide range of 1-MCP levels did not affect fruit respiration and ethylene production during storage of ‘Fuyu’ persimmon fruit (Kim and Lee, 2005). However, fruit respiration and ethylene production in the cultivar ‘Qiandaowuhe’ were delayed by 1-MCP treatment at ambient temperature (Luo, 2007). The fruit harvest time was much earlier in this study than the typical commercial harvest window, which occurs at the end of October (Yoo et al., 2016). Win et al. (2017) also reported an effect of harvest time on the different responses of ethylene production and respiration rates in cold-stored ‘Sangjudungsi’ persimmon fruit. Based on the results of two different harvest times, ethylene production and respiration rates were highly responsive during cold storage, irrespective of any postharvest treatments (Win et al., 2018). Nevertheless, ethylene production and respiration rates were more responsive in ‘Tonewase’ than in ‘Sangjudungsi’ during cold storage. It was assumed that ‘Tonewase’ fruit would be relatively less affected by fruit maturity. Otherwise, the sensitivity or responsiveness of fruit ripening would be much higher in ‘Tonewase’ than in ‘Sangjudungsi’. This would be due to differences in the types of astringent persimmon fruit (Yonemori et al., 2010), in which ‘Tonewase’ is a pollination variant astringent (PVA) type (Hamada et al., 2009) but ‘Sangjudungsi’ is a pollination constant astringent (PCA) type (Kim et al., 2018). Therefore, we considered that the different responses of ethylene production and respiration rates would be fruit-type dependent.
The combined application of the 1-MCP and deastringency treatments reduced the incidence of peel blackening compared with that of untreated fruit (Fig. 4). However, fruit decay and wilting were highest with the combination treatments, especially in ‘Tonewase’, during cold storage. This might be attributed to the difference in susceptibility of persimmon cultivars to chilling injury during cold storage. Collins and Tisdell (1995) reported that ‘Fuyu’ was more susceptible to chilling injury than ‘Suruga’ after cold storage, although the SSC was higher in ‘Fuyu’ than in ‘Suruga’, regardless of the incidence of chilling injury. The fruit decay index of ‘Rojo Brillante’ persimmon was marginally lower than that of ‘Triumph’ due to the single or combined application of a controlled atmosphere (CA) storage system or 1-MCP treatment during and after cold storage (Besada et al., 2014). The incidence of peel blackening and fruit softening during cold storage presented annual variation, regardless of treatments with 1-MCP and aminoethoxyvinylglycine in ‘Tonewase’ and ‘Sangjudungsi’ persimmons (Win et al., 2017; Win et al., 2018). Based on the different responses to the incidence of numerous physiological disorders, certain astringent persimmon cultivars, such as ‘Tonewase’, could be more susceptible to chilling injury than other cultivars.
In conclusion, deastringency treatments can be used to remove bitterness in astringent persimmon cultivars ‘Tonewase’ and ‘Sangjudungsi’. The time needed for complete removal of astringency differed by cultivar. In terms of retaining fruit quality during and after cold storage, the 1-MCP treatment alone was highly effective in maintaining fruit firmness in both cultivars during cold storage compared with that of the combined application of 1-MCP and deastringency treatments. However, the combined treatments would be more beneficial due to the depletion of tannin content during cold storage. Nonetheless, this combined treatment would be more beneficial in ‘Sangjudungsi’ than in ‘Tonewase’ in terms of reducing the incidence of physiological disorders, such as peel blackening, fruit decay, and wilting. Overall, the results indicate that each astringent persimmon cultivar should be treated with individually-tailored management techniques before cold storage to retain fruit quality.