Introduction
Materials and Methods
Plant Materials
PGR Application
Growth Conditions
Data Collection and Analysis
Results
Effects of BA and Ethephon Treatments on Growth Characteristics
Effect of BA and Ethephon Treatments on Crown Bud Formation
Discussion
Effects of BA and Ethephon Treatments on Growth Characteristics
Effect of BA and Ethephon Treatments on Crown Bud Formation
Introduction
Hosta Tratt. is a genus of the family Asparagaceae (The Angiosperm Phylogeny Group, 2009) and is an important genus for horticulture worldwide (Chung et al., 1991). The genus Hosta consists of approximately 22 - 25 species that are native to eastern Asia (mainly Korea and Japan) (Chung and Samuel, 1989; Chung et al., 1991). Among them, H. yingeri S.B.Jones is endemic to Korea (Chung and Chung, 1994), and differs from other Hosta taxa in having relatively thick, shiny, dark-green leaves and flowers spread evenly around the axis of the inflorescence (Kim et al., 2016). H. yingeri blooms in late summer from July to August (Korea National Arboretum, 2018). H. minor f. alba (Nakai) F. Maek is a form of H. minor (Baker) Nakai which is also endemic to Korea (Chung, 1994; Chung et al., 2017), and has white flowers (Jo and Kim, 2017). Hosta ‘White Edge’ was developed by ethyl methanesulfonate treatment of H. minor seeds and has a white color on the edge of the leaves (Kim et al., 2013); H. ‘White Edge’ was registered to the Korea Seed and Variety Service for commercialization in 2007. Both H. minor and H. minor f. alba bloom from June to July in a shady site (Korea National Arboretum, 2018). In their natural habitats, the three Hosta species grow or spread with long creeping rhizomes (Jo and Kim, 2017); however, the growth rate of offset formation is very low. These three Hosta taxa have unique characteristics, including raceme inflorescences, white flowers, or white leaf edges, and have high potential for development as commercial potted plants, garden plants, and ground-cover types.
Hosta are generally propagated by crown division, tissue culture, and/or seed (Wilson and Rajapakse, 2000; Gu et al., 2009). Crown buds develop on tissue within the storage stem/root region of the crown as separate units comprising several associated buds (Samarakoon et al., 2012). Crown division is one of the most practical propagation methods for Hosta in nurseries and by breeders. Therefore, in field production, crown development is of major importance (Leclerc et al., 2005). However, only a few shoots and crown buds can be obtained from one plant within a year. Tissue cultured explants are costly and frequently may not be true to type (Garner et al., 1997; Kim et al., 2016). Seeds can be obtained from many Hosta plantlets, but seed propagation does not produce a plant identical to the parent. Thus, division is a good choice for propagation of Hosta plants in nurseries (Dunwell, 1999).
Chemical plant growth regulators (PGRs) are frequently used to promote lateral branching and crown bud formation, and targeted species-specific applications can be designed (Currey et al., 2016). Benzyladenine (BA), a synthetic cytokinin, increases branching in herbaceous ornamental plants, alters the hormonal ratio of cytokinins to auxins in plants, and suppresses apical dominance (Cline, 1991; Martin and Singletary, 1999; Farris et al., 2009). BA has been used to stimulate the outgrowth of suppressed rhizomic buds in Hosta (Keever, 1994), and node segments of Thyrsostachys siamensis Gamble (Obsuwan et al., 2019). According to previous studies, BA can induce the outgrowth of offsets in Hosta, and the offsets formed can be removed from the mother plant within 30 d of BA application (Garner et al., 1998; Keever and Brass, 1998). Foliar application of BA at 3,000 mg·L-1 promoted offset formation in stock plants of Hosta ‘Francee’ and ‘Francee Williams’ (Garner et al., 1998; Schultz et al., 2000). Ethephon is a general use PGR and its mode of action differs from that of other PGRs. Ethephon is frequently used in the floriculture industry to inhibit reproductive bud development, reduce internode elongation, and promote branching (Glady et al., 2007). Ethephon application increased branching and the number of vegetative cuttings in herbaceous ornamental crops such as Coreopsis ‘Moonbeam’ and Chrysanthemum (Glady et al., 2007; Huh et al., 2007). However, BA and ethephon applications do not interact with all floricultural crops to initiate flower abortion and/or to initiate lateral branching; thus, species-specific responses must be investigated.
Although, there have been several previous studies examining methods of increasing crown bud formation for Hosta production, few studies have investigated crown bud formation using PGR treatment in native Korean Hosta taxa. This study aimed to determine the effects of BA and ethephon application timing and concentration on crown bud formation in three Hosta taxa, including one cultivar, which have low capacity to form many crown buds within a year.
Materials and Methods
Plant Materials
The three Hosta taxa selected for this study, H. yingeri, H. minor f. alba, and H. ‘White Edge,’ were excised from stock plants that were grown in the Useful Plant Resources Center, Korea National Arboretum. The three species were divided into uniform single-bud divisions on April 8, 2017, and were transplanted into 11.5 cm diameter containers (Type 5b pot; Hwasung Industry, Okcheon, Korea) filled with a commercial soilless substrate (Baroker; Seoul Bio, Eumseong, Korea) mixed with decomposed granite (Masato; Styrolution Korea Ltd., Ulsan, Korea) at a 2:1 ratio (v/v), respectively. The commercial substrate (Baroker) was composed of 68% coir dust, 15% peat moss, 7% perlite, 6% vermiculite, and 4% zeolite.
PGR Application
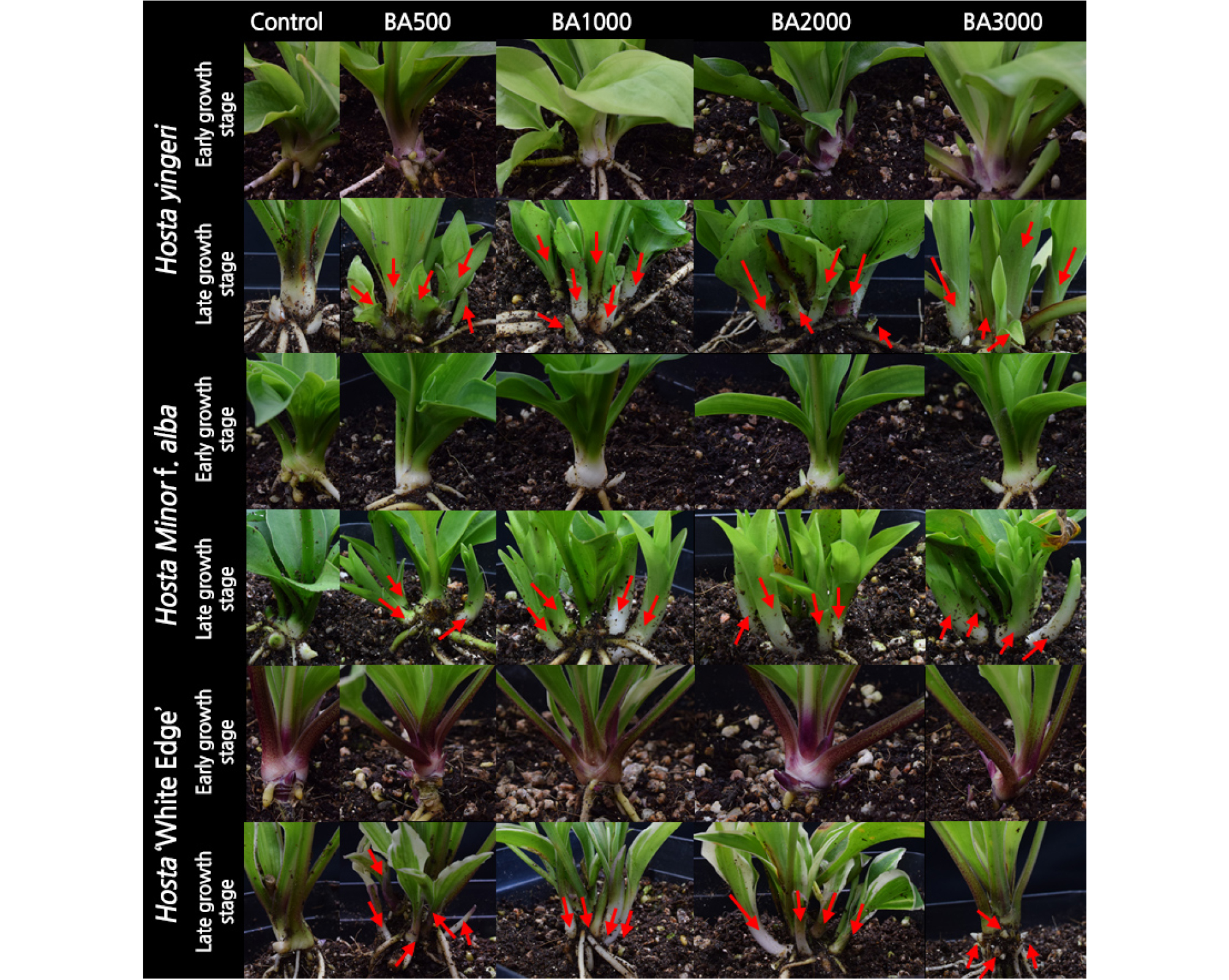
Single foliar sprays of 500, 1,000, 2,000, and 3,000 mg·L-1 BA (6-Benzylaminopurine; Duchefa, Haarlem, The Netherlands) and 50, 100, 500, and 1,000 mg·L-1 ethephon (Ethephon; Enbio, Gunpo, Korea) were applied to each plant at 10 mL per container using a hand-pump sprayer to uniformly wet the foliage and stems of all plants. BA and ethephon were applied in the morning. An untreated control was included for comparison. Spreader [Dong Bang (polyoxyethylene alkyl ether 10% + sodium ligno 20%); Dongbang Agro, Gwanak, Korea] at 0.06% was added to all spray solutions as a surfactant. There were two application timings: 1) early growth-stage treatment (May 29, 2017); 50 days after transplanting, and 2) late growth-stage treatment (September 1, 2017); 145 days after transplanting. Plants at the early growth-stage treatment had 2 to 3 leaves (5 - 6 cm long and 2 - 3 cm wide). Plants at the late growth-stage treatment had 8 to 9 leaves and at this stage the plants were already undergoing the process of leaf senescence.
Growth Conditions
The experiment was conducted between May 29, 2017 and April 5, 2018. The plants were placed in an outdoor nursery under a 55% shade cloth and irrigated by overhead sprinklers once daily during the growing season. Once per month from May to September plants were irrigated with 500 mg·L-1 of water-soluble fertilizer [Peters Professional (20%N, 20%P, 20%K, 0.05%Mg, 0.0125%B, 0.05%Fe, 0.025%Mn, 0.0125%Cu, 0.025%Zn, 0.005%Mo); Everris, Geldermalsem, The Netherlands] during vegetative growth. Plants were transferred to a greenhouse on November 23 and were maintained during the winter season; the average temperature in the greenhouse was approximately 5°C at 55% relative humidity. After the cold period during the winter, sprouting of the treated plants was observed on April 5, 2018.
Data Collection and Analysis
Plant height was measured from the soil level to the uppermost shoot and the plant width [(plant width at the widest point + width 90° to first width) / 2] was recorded. The number of leaves and crown buds were also counted. The growth parameters of the early growth stage were determined at 15, 40, 70, and 100 d after treatment (65, 90, 120, and 150 d after transplanting). The growth parameters for the late growth stage were determined at 15, 30, and 45 d after treatment (160, 175, and 190 d after transplanting). The number and shape of crown buds were also observed in treated plants on April 5, 2018, following the dormancy period. The experiment was conducted in a completely randomized design with 12 replicates (individual plants) for each treatment. Data were analyzed using SAS for Windows software (version 9.4; SAS Institute, Inc., Cary, NC, USA). The recorded data were analyzed by the analysis of variance (ANOVA) and Duncan’s multiple range test (p ≤ 0.05), and Pearson correlations of growth parameters such as number of crown buds, plant height, plant width, and number of leaves were calculated. Graph module analyses were performed using Sigma plot software (version 10.0; System Software, Inc., Chicago, IL, USA).
Results
Effects of BA and Ethephon Treatments on Growth Characteristics
In the early growth-stage treatment group, the response of growth parameters differed depending on the type of PGR, concentration, and species at 100 d after the PGR treatment (Table 1). The plant height of H. yingeri plants treated with 1,000 mg·L-1 ethephon was 1.4 cm shorter than that of the untreated plants. The width of H. ‘White Edge’ plants treated with 1,000 mg·L-1 and 3,000 mg·L-1 BA were 4.48 cm and 3.72 cm narrower, respectively, than those of the untreated plants. Also, H. ‘White Edge’ plants treated with 100 mg·L-1 and 1,000 mg·L-1 ethephon were 4.53 cm and 3.58 cm narrower, respectively, than the untreated plants. The number of leaves on H. yingeri plants increased significantly with 2,000 mg·L-1 and 3,000 mg·L-1 BA treatments compared with control plants (Table 1). The number of leaves on H. minor f. alba plants decreased with the 1,000 mg·L-1 ethephon treatment compared with control plants. The number of crown buds in the three Hosta species was strongly correlated with plant height, plant width, and number of leaves (Table 3).
Table 1. Effects of plant growth regulator treatments at an early growth stage on the growth parameters of three Hosta species. Plant growth regulator treatments were applied on May 29, 2017 and growth data were collected 100 days after treatment
NS,*,**Nonsignificant or significantly different at p ≤ 0.05 and 0.01, respectively.
In late growth-stage treatment group, there were no significant differences in plant height and plant width between H. yingeri and H. ‘White Edge’ at 30 d after the PGR treatments compared with control plants (Table 2). On the contrary, the plant height and plant width of H. minor f. alba were significantly increased by BA treatment compared with untreated plants, regardless of the concentration; the number of leaves also increased significantly after BA treatment regardless of concentration. PGRs and concentration interacted to affect the number of leaves of H. yingeri and H minor f. alba (Table 2). In H. yingeri, the average number of leaves increased from 4.8 to 30.3 with increasing BA concentration from 0 to 2,000 mg·L-1. In H. ‘White Edge’, the average number of leaves in plants treated with 1,000 and 2,000 mg·L-1 of BA was significantly higher than that in control plants, whereas the number of leaves were lower on plants that received ethephon treatments of 500 and 1,000 mg·L-1 (Table 2). Similar to the correlation results of the early growth-stage treatment group, the number of crown buds in late growth-stage treatments were strongly correlated with plant height, plant width, and number of leaves in the three Hosta species (Table 3).
Table 2. Effects of plant growth regulator treatments at a late growth stage on the growth parameters of three Hosta species. The plant growth regulators were applied on September 1, 2017 and growth data were collected 30 days after the treatments
NS,*,**,***Nonsignificant or significantly different at p ≤ 0.05, 0.01, and 0.001, respectively.
Table 3. Correlation coefficients between growth parameters and number of crown buds in three Hosta species
Effect of BA and Ethephon Treatments on Crown Bud Formation
The application of BA or ethephon increased the number of crown buds per plant in all three Hosta species (Fig. 1). However, the response to PGR application differed depending on the species, treatment timing, and concentration. In the case of H. yingeri, there was an average of 0.5 crown buds in untreated plants at 40 d after the early growth-stage treatment (Fig. 1). In the early growth-stage treatment, BA and ethephon treatments increased crown bud formation regardless of the concentration, compared with untreated plants. At 30 d after the late growth-stage treatment, there was an average of 1.4 crown buds in untreated plants. After application of 500, 1,000, 2,000, and 3,000 mg·L-1 BA, the average number of crown buds increased by 3.1, 5.3, 4.2, and 4, respectively, compared with the control plants. When 1,000 mg·L-1 BA was applied at the late growth stage, plants had 4.8-times as many crown buds as untreated plants. On the contrary, the number of crown buds in treated and untreated plants was not significantly different following ethephon application at late growth-stage treatment (Fig. 1). Furthermore, when H. yingeri was treated with BA, crown buds sprouted after 1,000, 2,000, and 3,000 mg·L-1 BA in the early growth-stage application and after all concentrations in the late growth-stage treatment at 15 days after treatments (Fig. 2).
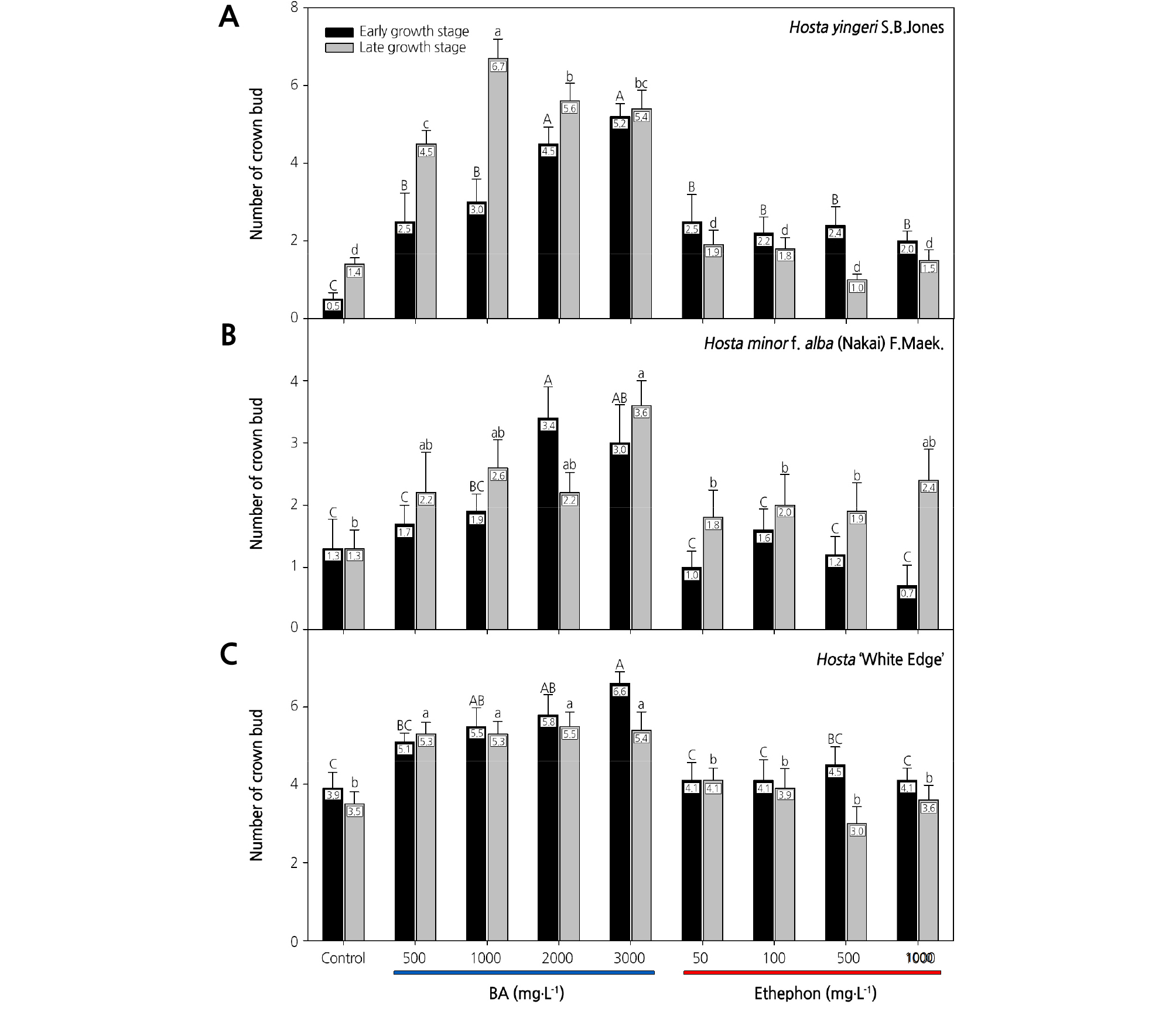
Fig. 1.
Effects of benzyladenine (BA) and ethephon treatments on the number of crown buds in three Hosta species. In Hosta yingeri (A) and Hosta minor f. alba (B), the number of crown buds was counted at 40 days after the early growth-stage treatments (on May 29, 2017) and at 30 days after the late growth-stage treatments (on September 1, 2017). In Hosta ‘White Edge’ (C), the number of crown buds was counted at 40 days after the early growth-stage treatments (on May 29, 2017) and at 15 days after the late-growth stage treatments (September 1, 2017). Letters above the columns indicate significant differences at p ≤ 0.05 (Duncan’s multiple range test).
H. minor f. alba had an average of 1.9 and 2.3 more crown buds than untreated plants after application of 2,000 mg·L-1 BA in the early growth-stage treatment and 3,000 mg·L-1 BA in the late growth-stage treatment, respectively (Fig. 1). Plants treated with 3,000 mg·L-1 BA at the late growth stage had 2.8-times as many crown buds as the untreated control plants. Ethephon treatments were not effective for promoting crown bud formation in H. minor f. alba. The crown buds formed had not sprouted in H. minor f. alba at 15 d after the early growth-stage treatment with ethephon, whereas they had sprouted following treatment with all BA concentrations at 15 d after the late growth-stage treatment (Fig. 2)
In the case of H. ‘White Edge,’ crown bud formation was enhanced to a greater degree by BA treatment than by ethephon treatment at both the early and late growth stages (Fig. 1). When 3,000 mg·L-1 BA was applied at the early growth stage, plants had 1.6-times as many crown buds as untreated control plants. The promotional effect of BA was not significantly different between the 1,000 and 3,000 mg·L-1 treatments at either the early or late growth stages. Crown buds sprouted at 15 d after the late growth-stage BA treatment (Fig. 2).
Discussion
Effects of BA and Ethephon Treatments on Growth Characteristics
The growth characteristics of the three Hosta species were affected by spray timing and concentration of the BA and ethephon treatments. In the early growth stage, ethephon application reduced plant height in H. yingeri and plant width in H. ‘White Edge’, although the effect of treatment concentration differed between the two species. Similar results have been reported in Angelonia and Verbena (Aiken et al., 2015; Currey et al., 2016). Currey et al. (2016) reported that Angelonia plant height was reduced with increasing ethephon concentration, from 22.0 cm (50 mg·L-1) to 19.2 cm (200 mg·L-1). Aiken et al. (2015) reported that Veronica plants treated with ethephon drenches were narrower than untreated plants, with no differences in plant height, whereas treated Verbena were consistently smaller (height and width) than untreated plants. In this study, the three Hosta species responded differently to ethephon treatments. Species-specific variation in the efficacy of ethephon treatments for plant growth parameters such as stem elongation, branch number, and plant width has also been reported in Angelonia and Geranium (Currey et al., 2016), and Kalanchoe (Currey and Erwin, 2012).
At the early growth stage, the effect of BA treatment on promoting leaf growth was only observed in H. yingeri among the three Hosta taxa in this study, whereas the promoting effect was observed in all Hosta species tested at the late growth stage. BA-induced promotion of lateral shoot development has been reported in many ornamental species such as Geranium (Carpenter and Carlson, 1972), Sandersonia aurantiaca (Davies et al., 1998), Ardisia pusilla (Lee, 2005), and Paeonia suffruticosa (Zhu et al., 2018). However, the response to BA treatment has been species-, or even cultivar-, dependent. H. yingeri grows at 2 - 300 m above sea level in remote islands off the southwestern coast of Korea, such as Taehuksan, Sohuksan, and Hong Islands, and grows mainly on rocky, open grasslands or talus slopes along the coast with several native species such as Hemerocallis hongdoensis and Pinus densiflora. (Chung and Chung, 1994). H. minor is found in primarily pine-oak forests in the middle eastern Korean Peninsula and southern parts of Gyeongsangnam and Chollanam Provinces, including Wan and Geoje Islands, and thus grows with naturally patchy distributions (Chung and Kim, 1991). In their natural habitats, H. yingeri has relatively fast-growing characteristics with thick, broad, and adaxially dark-green leaves in warm temperate regions compared to H. minor, which grows in temperate climate regions. Tworkoski and Miller (2007) reported that plants with different growth habits showed a different endogenous auxin:cytokinin ratio (ACR) and thus the plants had different responses to exogenous BA treatments. Miller and Eldridge (1986) also reported that apple (Malus domestica) cultivars with different growth habits have been found to respond differently to exogenous cytokinins.
In Sandersonia, branching was strongly promoted when tubers were soaked in 200 mg·L-1 BA solution (Davies et al., 1998). In Ardisia pusilla, the number of lateral shoots increased linearly with increasing BA concentration from 500 to 1,500 mg·L-1 (Lee, 2005). Variation in the efficacy of PGRs in promoting plant growth has been reported for Gladiolus sp. (Ram et al., 2002), Chrysanthemums (Huh et al., 2007), Saxifraga rosacea (Park et al., 2011), and Kalanchoe sp. (Currey and Erwin, 2012). Ram et al. (2002) also reported that treatment with 400 mg·L-1 ethephon significantly promoted cormel formation in Gladiolus sp., while BA was found to be less effective. An increase in offset formation after BA treatment was reported in Hosta (Keever, 1994; Schultz et al., 2000), whereas axillary buds in chrysanthemum ‘01B1-8’ increased by 17% with ethephon treatment (Huh et al., 2007).
Effect of BA and Ethephon Treatments on Crown Bud Formation
BA application was more effective than ethephon application at promoting crown bud formation in this study. BA effects on crown bud development in vivo have been reported in many plants such as Agastache, Lavandula, Leucanthemum (Grossman et al., 2012), Sedum spectabile ‘Autumn Joy’, Gaillardia aristata ‘Gallo Red’, Phlox paniculata ‘Bright Eyes’ (Grossman et al., 2013), Odontonema strictum ‘Red Fire spike’ (Rezazadeh et al., 2015), Chrysanthemum (Kim et al., 2003), and Malus (Yoon et al., 2000). BA is a synthetic cytokinin; cytokinins are plant hormones that promote cell division (Miller et al., 1955). In many species, the application of exogenous cytokinins enhances the ratio of cytokinin to auxin in the plant and promotes lateral bud outgrowth by disrupting apical dominance, which controls branching patterns and plant form (Cline, 1991). Keever (1994) found that in Hosta sieboldiana treated with a 2,000 and 3,000 mg·L-1 BA foliar spray, offset counts increased by 4.1 and 5.4 at 30 d after BA treatment, and that single-eye divisions were more likely to develop new offsets following BA foliar spray application than multiple offset plants. Several studies have indicated that ethephon promotes axillary budding in Chrysanthemums (Huh et al., 2007), Arabidopsis (Chatfield et al., 2000), Azalea, Fuchsia, Geranium, Lantana, and Verbena (Dole and Wilkins, 2005). In contrast, the application of ethephon had little or no influence on the growth of Hosta species in this study.
In our study, treatment at the late growth stage was more effective in promoting crown bud development in H. yingeri and H. minor f. alba than treatment at the early growth stage. The outcome of treatments at different growth stages is reportedly cultivar dependent. Garner et al. (1997) reported that treatment (on June 4) with 1,250 to 3,750 mg·L-1 BA was not effective for promoting offset formation in H. ‘Stilletto’ and H. ‘Mediovariegta’, however, offset formation increased in H. ‘Fragrant Blue’ and H. ‘Samuel Blue’. In another study, treatment (on August 20) with 3,000 mg·L-1 BA increased offset formation in H. ‘Sum & Substance’, H. ‘Elegans’, and H. ‘Francee’ (Schultz et al., 2000). In this study, late growth-stage BA treatments delayed leaf senescence and dormancy induction (data not shown). Zwack and Rashotte (2013) reported that cytokinin generally tends to have a delaying effect on leaf senescence. Low contents of endogenous cytokinins during the dormancy induction period were reported in Willow (Alvim et al., 1976) and in Astilbe chinensis var. davidii (Lee et al., 2006). We applied BA in early September for the late growth-stage treatments. Thus, the efficacy on promoting crown bud formation of the late growth-stage treatments might be due to the increase of cytokinin contents. Sato and Mori (2001) reported that auxin has an inhibitory effect in the growth of axillary buds, whereas cytokinin enhances axillary bud outgrowth, but the axillary bud outgrowth depends on the ratio of the two hormones rather than the absolute contents of either hormone. Hartmann et al. (2011) also reported that 6-benzylaminopurine (BA) application induced higher rates of bud break, and thus reactivation of meristem activity and sprout growth in potato tubers requires cytokinin. In addition, it appears that responses to different BA concentrations are species specific. A study of several maize (Zea mays) hybrids showed that plant height, stem diameter, and biomass production were stimulated considerably after the application of 50 or 100 mg·L-1 BA (Amin et al., 2007). Similar results were also observed in bean (Phaseolus vulgaris L. ‘Red kidney’) after leaf application of 30 mg·L-1 BA (Adedipe et al., 1971).
A significant correlation was observed between the number of crown buds and plant height, plant width, and number of leaves in this study, presumably due to the fact that the leaf number of the plants increased by sprouting of the crown buds. Schultz et al. (2000) reported that as offset number increases, quality rating and growth index [(height + width at the widest point + width 90° to first width)/3] increase in Hosta cultivars. Another previous study reported that the growth index was higher for treated plants than for controls of H. ‘Patriot’, and the growth index was increased linearly with increasing offset number at 60 days after treatment (Garner et al., 1997). Therefore, plant height, plant width, and number of leaves were increased by offset number and crown buds, and thus, plant appearance was enhanced by crown buds.
In addition, the persistence of the branching effect of BA can be affected by the frequency of application. Grossman et al. (2012) showed that Gaura and Lavandula plants showed continued improvements in branching as a result of multiple BA treatments during four weeks of growth. Therefore, further sequential applications may be necessary to promote a more positive response. PGR application using a substrate drench also showed more uniform effects and increased the duration of efficacy compared with foliar sprays (Gent and McAvoy, 2000; Boldt, 2008). Although foliar sprays were used in this study, future studies could examine plants treated using the drench method and compare the results to those of this study.
Based on the results of this study, it can be concluded that the efficacy of BA and ethephon application for crown bud development differs depending on the treatment timing and PGR type, and that a late growth-stage application of BA is more effective than an early growth-stage application. The results could contribute to the propagation of native Hosta species and to the development of new cultivars.