Introduction
Material and Methods
Experimental Area Characterization
Experimental Design and Plant Material
Experimental Conduction
Analyzed Variables
DOP Index
Data Analysis
Results
Seedling Dry Matter
Nutritional Status of Seedlings
Deviation from the Optimum Percentage (DOP)
Principal Component Analysis
Discussion
Dry Matter of Seedlings
Nutritional Status of Seedlings
Deviation from the Optimum Percentage
Principal Component Analysis
Conclusions
Introduction
Mango (Mangifera indica L.) is a species belonging to the family Anacardiaceae. In the world’s fruit production chain, it is grown in more than 80 tropical and subtropical countries, with production of 30 million tons in 2019 (FAO, 2020). In Brazil, mango is the fifth most commonly produced fruit and first in terms of exports (Kist et al., 2022). Among the producing states, Pernambuco and Bahia stand out (IBGE, 2022), with the São Francisco Valley region being the main producer and exporter of the country, accounting for 87% of exports (COMEX STAT, 2021). Among the most produced cultivars in the region, ‘Palmer’, ‘Tommy Atkins’, ‘Keitt’, and ‘Kent’ stand out (Kist et al., 2022). Despite their consolidation in the international market, Brazilian producers are, currently, in pursuit of new opportunities for international market windows by exploring new cultivars, mainly mangoes of Israeli origin, such as ‘Shelly’, ‘Omer’, and ‘Agam’.
Mango seedlings are commercially propagated by grafting (Pinto et al., 2018). However, little is known about the selection and use of suitable rootstocks for producing quality grafted seedlings, mainly in terms of efficiency in the absorption and translocation of nutrients to the scion. These factors, consequently, influence biomass conversion (Menegatti et al., 2022). Among the characteristics used to select rootstocks, root development factors that promote more efficient mechanisms for capturing and translocating water and nutrients to the scion have been noted, enhancing seedling growth and nutrition (Hayat et al., 2021).
In related research, many studies have highlighted the influence of rootstocks on the acquisition of nutrients from the soil and their transport to the scion (Valverdi and Kalcsits, 2021). Such an effect depends on mechanisms related to rootstock-scion communication. It is bidirectional through the xylem and phloem, including responses in the absorption of water and nutrients, the hormonal balance, and the synthesis of metabolites and nucleic acids (Albacete et al., 2015). Nutrient transport through rootstock-scion binding is also dependent on genetic factors and compatibility between plant materials. It may also increase plant hydraulic capacity and shoot nutrient accumulation levels.
Rootstock effects on the canopy nutritional status are well described in the literature for several fruit crops, such as peach - Prunus persica (Efstathios et al., 2021), pear - Pyrus communis L. (Nazli and Erdal, 2019), citrus - Citrus sinensis [L.] Osb. × Poncirus trifoliata and Citrus reshni Hort. ex Tanaka (Caballero et al., 2013), grapes - Vitis vinifera (Tomasi et al., 2015), and atemoya - Annona × atemoya (Baron et al., 2018). Mainly for mangoes, data on the influence of rootstock on shoot mineral nutrition in the available literature is quite limited. An example is Zayan et al. (2020), who evaluated the influence of four rootstocks on the mineral nutrition of the mango var. Naomi. Likewise, Sarkhosh et al. (2021) verified the influence of rootstock on the mineral composition of scions from “B74” and “KP” mango seedlings.
Based on the above, rootstock/scion interactions may induce seedling vigor caused by exogenous (water and nutrients) and endogenous (hormones and secondary metabolites) factors that move across the graft site binding (Albacete et al., 2015). Understanding this process in relation to seedling vigor and scion nutrition with Israeli mango recently introduced in the São Francisco Valley region can contribute to the selection of the most suitable rootstocks for these cultivars and to the planning of a more assertive supply of nutrients based on the specificity of each material (Tomasi et al., 2015).
This study hypothesized a direct rootstock effect on the scion mineral nutrition of the scion cultivar in several fruit crops and even in mango seedlings; indeed, the rootstock/scion combination most likely determines the choice of rootstock(s) that will provide balanced mineral nutrition for seedlings of the cultivars ‘Shelly’, ‘Agam’ and ‘Omer’ in the semi-arid conditions of the São Francisco Valley of Brazil. This information is new in the scientific literature given that scion × rootstocks combinations evaluations have not been mentioned thus far, including seedling formation, which is the first step in forming a new orchard. In this sense, a study was carried out to evaluate the dry matter production and nutritional status of Israeli mango seedlings (‘Shelly’, ‘Omer’, and ‘Agam’) grafted onto ‘Coquinho’ and ‘Espada’ rootstocks.
Material and Methods
Experimental Area Characterization
The experiment was carried out between December of 2020 and April of 2021 in the fruit nursery of the Agricultural Sciences Campus of the Federal University of São Francisco (UNIVASF). It is located in the Nilo Coelho irrigated perimeter, in Petrolina, Pernambuco State, Brazil (9° 09’ S, 40º 22’ W, and 365.5-m above sea level). The region’s climate is classified as BSh, indicating a hot semi-arid climate (Alvares et al., 2013) with average annual rainfall of 481.7 mm and air temperatures ranging from 24 to 28°C in the region of the sub-medium San Francisco Valley.
During the experiment, meteorological data pertaining to rainfall, temperatures (maximum, minimum, and average), relative humidity levels, and global solar radiation were recorded at an automatic meteorological station at UNIVASF, installed near the experimental area on the Campus of Agricultural Sciences (Fig. 1).
Experimental Design and Plant Material
The experiment was carried out in randomized blocks, arranged in a 3 × 2 factorial scheme, corresponding to three Israeli mango cultivars denoted as scion–Sc (‘Agam’, ‘Omer’, and ‘Shelly’) and two mango cultivars denoted as rootstock - Rt (‘Espada’ and ‘Coquinho’), with three replications and ten plants per plot. The rootstocks ‘Espada’ and ‘Coquinho’ were obtained from commercial nurseries located, respectively, in the cities of Petrolina (PE) and Curaçá (BA) registered with the Ministry of Supply, Livestock, and Agriculture (MAPA). The scions ‘Agam’, ‘Omer’, and ‘Shelly’ were obtained from mother plants located in the city of Petrolina (PE).
Experimental Conduction
Rootstock seedlings were obtained from seeds of mature fruits of the cultivars ‘Coquinho’ and ‘Espada’. After cleaning and shade drying, seeds were sown in 1-L polyethylene bags filled with sand and kept in a protected environment until grafting. Seedlings were prepared using cleft grafting, following the technical recommendations of Genú and Pinto (2002). Grafted seedlings were kept in a protected environment under 50% shading and were irrigated daily using an inverted localized micro-sprinkler, with 45 L·h-1 sprinklers.
The grafted seedlings were fertilized (via foliar with fertilizers containing 10% nitrogen, 8% phosphorus (P2O5), 8% potassium (K2O), 1% calcium (Ca), 0.5% magnesium (Mg), 0.5% boron (B), 0.2% copper (Cu), 0.5% manganese (Mn) and zinc (Zn), applied fortnightly) and were treated with a nutritional fertigation solution (containing 0.2 g of urea (45% N ), 0.15 g of potassium sulfate (51% K), 0.15 g of monoammonium phosphate (48% P2O5) and 0.25 mL of fulvic acid in 1 L of water, applied every 3 days).
Analyzed Variables
At 150 days after grafting (DAG), seedlings were measured for dry matter (Fig. 2). Plants were separated into leaves (LDM), stems (SDM), and roots (RDM), taken to the laboratory, washed in distilled water and then placed in an air circulation oven at 65°C until a constant mass was recorded. The dry mass of each part was then determined by weighing it on a semi-analytical scale (precision <0.01 g). Total dry matter (TDM) was then obtained by summing LDM, SDM, and RDM.
In the same period, leaf samples were collected to analyze nutritional status, following the criteria of Malavolta et al. (1997). The samples were packed in paper bags, identified, and taken to the Laboratory of Soil Chemistry and Fertility at UNIVASF (Fig. 2). After washing with distilled water, leaves were dried in an oven with air circulation at 60 ºC until a constant weight was reached, after which they were ground in a stainless-steel knife mill (Willey type) and stored in an airtight container. Contents of macronutrients [nitrogen (N), potassium (K), phosphorus (P), calcium (Ca) and magnesium (Mg)] and micronutrients [copper (Cu), iron (Fe), manganese (Mn) and zinc (Zn)] and sodium (Na) in the mango leaves were determined according to methods described by Silva (2009).
DOP Index
The index known as deviation from optimal percentage (DOP) was calculated by diagnosing leaf nutritional status according to Heras and Montañés (1991). The DOP index was estimated for an analysis of the leaf mineral composition using the formula DOP = [(C × 100)/Cref]–100, wherein C denotes the concentration in the sample to be studied and Cref is the concentration considered optimal for the nutrient. Cref was proposed based on optimum values for mango seedlings, as determined by Reuter (1997), included in Table 1. Negative DOP values indicate nutrient deficiency, while positive values indicate excess, with a null value representing a balanced condition of the nutrient (Kumar et al., 2017a).
Table 1.
Reference concentrations (Cref) of the macronutrients (N, P, K, Ca and Mg) considered optimal for mango seedlings for the calculation of the deviation to optimal percentage (DOP) index
| Nutrients | Reference concentrations (Cref) | ||||
| N | P | K | Ca | Mg | |
| - g·k-1 - | |||||
| 18.7 | 1.4 | 9.4 | 32.0 | 3.2 | |
Reference concentration considered optimal by mango seedlings according to Reuter (1997).
Data Analysis
Data were subjected to an analysis of variance (ANOVA) by an F-test at 5% probability. Averages for rootstocks and scions were compared by Tukey’s test at 5% probability. Analyses were conducted using the statistical software SISVAR, version 5.6 (Ferreira, 2019). A principal component analysis (PCA) was also conducted to verify rootstock/scion interrelationships using the statistical software R (Team, 2017).
Results
Seedling Dry Matter
The rootstock × scion interaction had no significant effect on dry matter outcomes, with only the rootstock having an effect (Table 2). Mango seedlings of the Israeli cultivars showed greater vigor with the rootstock ‘Espada’, presenting higher dry matter values in relation to those of the seedlings with ‘Coquinho’. According to the values determined, the rootstock ‘Espada’ promoted increases of 419.36%, 256.45%, 268.37%, and 280.52% for LDM, SDM, RDM, and TDM, respectively.
Table 2.
Summary of the analysis of variance according to the ‘F’ values for the leaf dry matter (LDM), stem dry matter (SDM), root dry matter (RDM) and total dry matter (TDM) of mango seedlings as a function of different rootstocks (Rt) and scions (Sc) of Israeli mango cultivars
| Sources of variation | LDM | SDM | RDM | TDM |
| - g - | ||||
| Rootstock | 151.13** | 189.00** | 23.19** | 88.69** |
| ‘Espada’ | 19.92 a | 46.93 a | 48.12 a | 114.96 a |
| ‘Coquinho’ | 4.75 b | 18.30 b | 17.93 b | 40.98 b |
| Scion | 2.32ns | 2.83ns | 1.65ns | 2.69ns |
| ‘Omer’ | 12.65 | 31.04 | 27.84 | 71.52 |
| ‘Agam’ | 13.78 | 36.11 | 40.96 | 90.85 |
| ‘Shelly’ | 10.57 | 30.69 | 30.28 | 71.52 |
| Rt × Sc | 1.16ns | 0.11ns | 0.001 ns | 0.064ns |
| Mean | 12.33 | 32.61 | 33.02 | 77.97 |
| CV (%) | 24.50 | 15.64 | 46.48 | 24.68 |
ns: not significant according to Tukey’s test at 5% probability; **: significant according to Tukey’s test at 1% probability; *: significant according to Tukey’s test at 5% probability; CV: coefficient of variation. LDM = leaf dry matter, SDM = stem dry matter, RDM = root dry matter, TDM = total dry matter.
Nutritional Status of Seedlings
Table 2 shows that the Rt × Sc interaction promoted a significant effect on potassium (K) and phosphorus (P) leaf contents. Nitrogen (N), calcium (Ca), and magnesium (Mg) leaf contents did not respond to the treatments and showed corresponding averages of 13.66 g·kg-1, 27.78 g·kg-1, and 3.62 g·kg-1. Among the cultivars, ‘Agam’ and ‘Shelly’ mango seedlings showed the highest leaf contents of K, with these levels not statistically differing from each other. With regard to the mango seedlings cv. ‘Omer’, the leaf contents K of the ‘Agam’ and ‘Shelly’ cultivars were higher by 22.89% and 17.55%, respectively (Table 3).
Table 3.
Summary of the analysis of variance according to the ‘F’ values for nutritional status in macronutrients [nitrogen (N), potassium (K), phosphorus (P), calcium (Ca) and magnesium (Mg)] in mango leaves as a function of different rootstocks (Rt) and scions (Sc) of Israeli mango cultivars
| Source of variation | N | K | P | Ca | Mg |
| - g·kg-1 - | |||||
| Rootstock | 0.05ns | 4.39ns | 1.43ns | 0.15ns | 0.95ns |
| ‘Espada’ | 13.73 | 7.74 | 1.56 | 28.39 | 3.76 |
| ‘Coquinho’ | 13.60 | 8.41 | 1.42 | 27.18 | 3.49 |
| Scion | 0.72ns | 9.48** | 0.43ns | 0.60ns | 1.82ns |
| ‘Omer’ | 13.32 | 7.12 b | 1.47 | 25.45 | 3.25 |
| ‘Agam’ | 13.53 | 8.75 a | 1.45 | 28.45 | 3.79 |
| ‘Shelly’ | 14.14 | 8.37 a | 1.59 | 29.46 | 3.82 |
| Rt × Sc | 0.84ns | 29.19** | 7.64** | 0.52ns | 2.12ns |
| Mean | 13.66 | 8.07 | 1.50 | 27.78 | 3.62 |
| CV (%) | 10.40 | 9.67 | 20.82 | 27.38 | 18.50 |
The leaf contents of K and P showed different outcomes as a function of the rootstock/scion combination (Fig. 2A and 2B). The rootstock ‘Coquinho’ promoted the highest leaf K content with the scion cultivars ‘Agam’ (20% higher than ‘Espada’) and ‘Shelly’ (35% higher than ‘Espada’), while for the scion cultivar ‘Omer’, the highest leaf K content was promoted by the ‘Espada’ rootstock, which was 29.3% higher than ‘Coquinho’ (Fig. 3A). Regarding the P foliar contents, an inverse data distribution was observed (Fig. 3B); i.e. the rootstock ‘Espada’ had the highest P content in the mango seedlings of cultivars ‘Agam’ and ‘Shelly’, with average values nearly 37% and 17.6% higher than those of ‘Coquinho’, respectively, while the scion cultivar ‘Omer’ presented P leaf contents 31.4% higher with the ‘Coquinho’ rootstock.

Fig. 3.
Leaf contents of potassium - K (A), phosphorus - P (B) in the Israeli mango seedlings ‘Agam’, ‘Omer’, and ‘Shelly’ as a function of the rootstocks ‘Espada’ and ‘Coquinho’. Bars with the same lowercase letters do not differ from each other for the rootstocks ‘Espada’ and ‘Coquinho’ within each Israeli mango cultivar as scions according to F tests (p < 0.05). Bars with the same capital letters do not differ from each other for the ‘Agam’, ‘Omer’, and ‘Shelly’ Israeli mango scions within each rootstock according to Tukey’s test (p < 0.05).
According to the ANOVA summary (Table 4), the interaction between rootstocks and mango scions influenced only the leaf copper (Cu) content. The leaf iron (Fe) content responded to rootstocks, the leaf zinc (Zn) content to scion varieties, and the sodium (Na) content to both factors separately. The leaf manganese (Mn) content was not influenced by the treatments and showed an average content of 173.43 mg·kg-1 dry matter.
Table 4.
Summary of analysis of variance according to the ‘F’ values for nutritional status in micronutrients [copper (Cu), iron (Fe), manganese (Mn) and zinc (Zn)] and sodium (Na) in mango leaves as a function of different rootstocks (Rt) and scions (Sc) of Israeli mango cultivars
| Source of variation | Mn | Cu | Fe | Zn | Na |
| - mg·kg-1 - | |||||
| Rootstock | 0.14ns | 3.99ns | 5.41* | 3.01ns | 17.67** |
| ‘Espada’ | 169.51 | 18.38 | 61.87 b | 50.46 | 158.19 b |
| ‘Coquinho’ | 177.37 | 16.06 | 78.34 a | 56.92 | 204.02 a |
| Scion | 1.72ns | 2.03ns | 0.248ns | 4.93* | 8.29** |
| ‘Omer’ | 149.03 | 17.19 | 72.96 | 51.41 ab | 168.63 b |
| ‘Agam’ | 173.99 | 15.80 | 70.48 | 47.94 b | 162.40 b |
| ‘Shelly’ | 197.29 | 18.67 | 66.88 | 61.71 s | 212.28 a |
| Rt × Sc | 0.09ns | 12.23** | 0.64ns | 3.22ns | 1.90ns |
| Mean | 173.43 | 17.22 | 70.10 | 53.68 | 181.10 |
| CV (%) | 29.99 | 16.54 | 24.74 | 17.00 | 14.74 |
According to Table 4, mango seedlings grafted onto ‘Coquinho’ rootstock led to increased Fe and Na leaf contents, with increments of 26.62% and 28.97%, respectively, when compared to those grafted onto ‘Espada’. Among the Israeli cultivars, ‘Shelly’ showed higher Zn and Na leaf contents compared to ‘Omer’ and ‘Agam’, but the Zn leaf contents showed no difference for the mango cv. ‘Omer’.
The ‘Coquinho’ rootstock showed increased leaf copper (Cu) contents for the scions ‘Omer’ and ‘Shelly’, while the ‘Espada’ rootstock showed the highest Cu leaf content in the scion ‘Agam’ (Fig. 4).
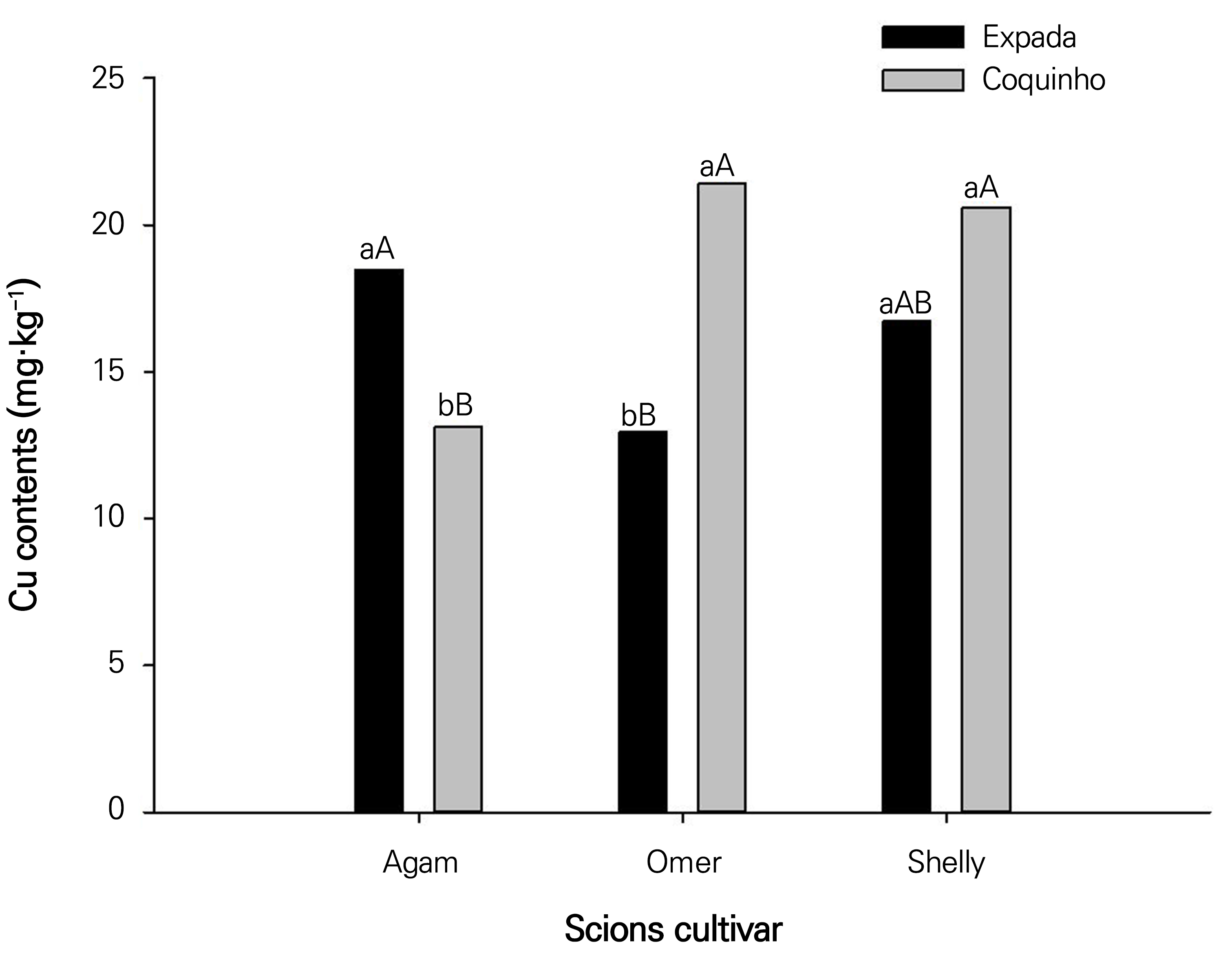
Fig. 4.
Leaf copper content - Cu in ‘Agam’, ‘Omer’, and ‘Shelly’ Israeli mango seedlings as a function of the rootstocks ‘Espada’ and ‘Coquinho’. Bars with the same lowercase letters do not differ from each other for the rootstocks ‘Espada’ and ‘Coquinho’ within each Israeli mango cultivar as scions according to F-tests (p < 0.05). Bars with the same capital letters do not differ from each other for scions of the Israeli mango cultivars ‘Agam’, ‘Omer’, and ‘Shelly’ for each rootstock according to Tukey’s test (p < 0.05).
Deviation from the Optimum Percentage (DOP)
Table 5 shows that the highest positive values of the DOP indexes were for the macronutrients P and Mg in seedlings with the ‘Espada’ rootstock and ‘Agam’ scion, indicating that the levels of elements are above those considered ideal for mango seedlings. The mango seedlings with the rootstock ‘Coquinho’ and scion ‘Omer’ had P and Mg values closer to those considered optimal according to the DOP index. For all rootstock-scion combinations, the DOP indexes for N, K, and Ca were more negative, highlighting their deficiency in these nutrients. The highest N deficiency in seedlings was observed for the ‘Coquinho’ rootstock combined with the cultivar ‘Omer’, while for K and Mg it was recorded for the ‘Espada’ rootstock with the ‘Omer’ scion. These findings indicate that the seedlings of the mango ‘Omer’ as a scion had the lowest compatibility with all rootstocks studied, increasing the macronutrient imbalance.
Table 5.
Impacts of rootstock and Israeli mango scion combinations on the deviation from optimum percentage (DOP index) of macronutrients [nitrogen (N), potassium (K), phosphorus (P), calcium (Ca) and magnesium (Mg)] in seedlings
Principal Component Analysis
Based on the dry matter accumulation outcomes and the nutritional status (macronutrients and micronutrients) of mango seedlings, a principal component analysis (PCA) identified two groups defined by separation between the ‘Espada’ and ‘Coquinho’ rootstocks, with the responses varying with the rootstock and scion combination (Fig. 5). The eigenvalues of the first and second components were, respectively, 44.2 and 31.7%, with an accumulation rate of 75.9% for cumulative eigenvalues from the variance data. In PC1, there was a high positive correlation between the dry matter and Ca, while Fe and Na showed a high negative correlation with the dry matter. PC2 showed high positive inter-correlations for K, Fe, and Na.
The combinations ‘Espada’ and ‘Agam’ and, mainly, ‘Espada’ and ‘Omer’ tended to have higher dry matter accumulation rates and Ca leaf contents (Fig. 5). The combination ‘Coquinho’ with the ‘Omer’ and ‘Shelly’ scions showed higher levels of micronutrients and Na, but the seedlings tended to accumulate less dry matter. The combination ‘Coquinho’ and ‘Agam’ induced a higher K leaf content, with antagonistic behavior toward N, Ca, and Mg.
Discussion
Dry Matter of Seedlings
The rootstock ‘Espada’ promoted a higher dry matter accumulation level in seedlings grafted onto the Israeli cultivars, with greater increments of the leaf and root dry masses (Table 2). Changes in the root morphological characteristics of grafted mango seedlings can be attributed to genetic differences linked to environmental changes and agricultural practices (Kumar et al., 2017b). Similar behavior was observed in ‘Red Fuji’ apple trees by Hayat et al. (2020), who reported differences in root traits for the rootstocks evaluated, with superior values of the root volume and length in the cultivar ‘Baleng’. They attributed such differences to genetic alterations.
The greater vigor of mango seedlings grafted onto ‘Espada’ rootstock may be related to genetic factors of the cultivar, which may have promoted changes in molecular and physiological aspects between the rootstock and scion (Nazli and Erdal, 2019). By allocating greater dry matter production to the roots, the absorption and transport of mineral ions increased, which in turn enhanced the shoot dry matter level (Menegatti et al., 2022). This result is in line with that of Hayat et al. (2021), who concluded that rootstocks with smaller root systems (root volume, root diameter, number of roots) have a lower capacity to absorb water and nutrients from the soil.
Shoot dry mass indicates seedling rusticity, in which the higher the content, the greater the degrees of rusticity and lignification. Conversely, root dry matter is one of the best and most important variables to estimate the survival and initial growth of seedlings in the field (Gomes and Paiva, 2011). Rebolledo-Martínez et al. (2019) also verified this behavior when evaluating the effect of the cultivar ‘Manila’ on seven rootstocks; they observed that dry matter was positively affected by rootstocks, with better results for the cultivars ‘Criollo’, ‘Julie’, ‘Gomera 1’, ‘Esmeralda’, and ‘Thomas’. In contrast, Sarkhosh et al. (2021) concluded that scions had an effect on leaf dry matter accumulation for commercial mango cultivars and the National Mango Board breeding program evaluated in Australia, but showed no difference between rootstocks with polyembryonic mango genotypes.
Nutritional Status of Seedlings
In our research, rootstocks × scion interactions interfered with K, P, and Cu leaf contents, but the responses were more pronounced for other nutrients when treatments were applied in isolation. In mango, some authors have confirmed the influence of rootstocks on the absorption of nutrients and on the nutritional status in plants (Duran-Zuazo et al., 2004; Zuazo and Tarifa, 2006; Zayan et al., 2020; Sarkhosh et al., 2021). The capacity of the rootstock to absorb water and nutrients varies with the root architecture and/or more efficient intake or transport mechanisms, which are characteristics that regulate plant growth (Hayat et al., 2020; Hayat et al., 2021). This statement is also highlighted by Kviklys et al. (2017), who concluded that the rootstock effect on the leaf mineral composition is related to its ability to absorb nutrients given its specific root morphology. Nutrient uptake and accumulation are related to genetic and phenotypic traits of scions, as well as rootstocks, which may have greater hydraulic conductance, showing a positive correlation with nutrient accumulation in scions (Sharma et al., 2016).
The leaf mineral compositions of K, P, and Cu varied with the rootstock/scion combinations. This may have arisen due to genetic material differences in terms of the absorption of these nutrients by rootstocks and their use by the scion. These differences are governed by specific genes and their interactions in each rootstock/scion combination (Kumar et al., 2017b; Sharma et al., 2016).
In this sense, the rootstocks influence nutritional status dynamics due to changes in the root distribution, affecting mineral absorption and potential variations in the anatomical structures. These characteristics are related to the ability of the hydraulic state to supply nutrients through the roots to the leaves (Zarrouk et al., 2005; Hayat et al., 2020; Hayat et al., 2021). Although rootstocks affect the leaf mineral composition, this organ belongs to the scions, which influence the leaf composition (Lazare et al., 2020). For example, the similar responses of ‘Agam’ and ‘Shelly’ can be explained by the genetic proximity of both. According to Cohen et al. (2016), the cultivar ‘Shelly’ is the result of a cross between the cultivars ‘Tommy Atkins’ and ‘Kent’, while ‘Agam’ is the result of open pollination of the cultivar ‘Shelly’
Table 3 shows the average leaf contents of N, Ca, and Mg. Although the treatments had no effect, the levels are within the optimum range determined by Quaggio (1996) for mangoes (N–12.0 to 14.0 g·kg-1; Ca–20.0 to 35.0 g·kg-1, and Mg–2.5 to 5.0 g·kg-1). However, for high-yield mango trees (Rezende et al., 2022), seedlings were deficient in N and supplied with Ca and Mg. It should be noted that despite comparing the nutrients using different authors’ findings, the values refer to older plants (full production) and are due to a lack of information about the proper nutritional status of mango seedlings and the evaluated cultivars.
Similar behavior was recorded for other fruit trees, including mango, in which the rootstock and scion combination influences the mineral compositions of N, Ca, and Mg. The leaf nutritional status of commercial mango cultivars grown in Australia showed no response to rootstock/scion combinations, with outcomes ranging from 13.5 to 14.8 g·kg-1. The authors attributed this to rootstock similarities in the root architecture, cation exchange, root exudate, and N absorption capacity. Leaf N levels were lower than those found by Zayan et al. (2020) in one-year-old ‘Naomi’ mangoes grafted onto the rootstocks ‘Socaria’, ‘Gomera-3’, ‘Sabre’, and ‘Hybrid 13/1’, varying between 15.6 and 19.7 g N kg-1.
Valverdi and Kalcsits (2021) also highlighted the lack of a rootstock response when studying the foliar Ca contents in apple trees (Malus domestica) of the cultivar ‘Honeycrisp’. However, when assessing nine citrus (Citrus paradisi Macf.) rootstocks, Sharma et al. (2016) found differences in Ca contents for ‘Attani-2’, ‘Laranja Azeda’, and ‘Jatti Khatti’ in ‘Marsh Seedless’ as well as ‘Troyer’ in ‘Redblush’; such outcomes with regard to leaf contents may be due to genetics and to the phenotypic proximity of scions with Israeli mangoes in the evaluated rootstocks. Our findings with regard to leaf Mg contents are superior to those of Zuazo and Tarifa (2006), who reported a range of 1.5 and 2.5 g·kg-1 and are close to those observed by Sarkhosh et al. (2021), who found values from 2.6 to 3.6 g·kg-1.
We found leaf K contents higher than those reported by Zuazo and Tarifa (2006). These authors studied the effect of four rootstocks on the mineral nutrition of the cultivar ‘Keitt’ and observed that the rootstock/scion interaction responded, with values ranging from 5.0 to 6.0 g·kg-1. In mango, K is one of the nutrients most extracted and exported by plants (Silva et al., 2002), and it is involved in the synthesis of starch in the leaves, photosynthetic activity, the transport of carbohydrates, the circulation of sap, and plant water and osmotic regulation (Silva et al., 2002; Marschner, 2012).
The leaf P contents varied with the rootstock/scion combination, with higher values for Espada/Agam, Espada/Shelly, and Coquinho/Omer (Fig. 3B). This characteristic prevents the identification of which material stood out and can be explained in terms of the substrate’s ability to provide nutrients to plants (Nazli and Erdal, 2019). Because seedlings were within adequate levels according to recommendations by Quaggio (1996) (from 0.8 to 1.6 g·kg-1) and Rezende et al. (2022) (from 1.5 to 2.3 g·kg-1), they did not reach excessive levels (>2.5 g·kg-1) (Mouco, 2010).
Zayan et al. (2020) also observed significant effects of rootstock/scion interaction on the leaf P content for ‘Naomi’ mango, with a superior rate of 18.9% when using the ‘Socaria’ rootstock compared to the rootstock ‘Hibrido 13/1’. As phosphorus is considered practically immobile in the soil (Mardamootoo et al., 2021), the rootstock ‘Espada’ can provide a relative advantage with regard to its acquisition, as this cultivar has a more robust and developed root system compared to that of ‘Coquinho’. However, compatibility between materials must be considered, as this result was not repeated for ‘Espada’/‘Omer’, with superiority found for the rootstock ‘Coquinho’.
Leaf Mn and Fe contents did not respond to the treatment application, but Israeli mango seedlings exceeded the optimal level and were adequately supplied, respectively, according to adequate Mn (50 to 100 mg·kg-1) and Fe (50 to 200 mg·kg-1) amounts defined by Quaggio (1996).
Our findings with regard to leaf Cu contents were within the suitable range according to the classification by Quaggio (1996) (>10 mg·kg-1). This result is surprising when considering that for all dry matter partitioning variables, the cultivar ‘Espada’ was superior to ‘Coquinho’, which characterizes rootstock specificity. By evaluating the origin of phenotypic variations of mango cultivars with five samples of ‘Espada’, two of ‘Coquinho’, and two of ‘Comum’, using RAPD molecular marker, Faleiro et al. (2012) observed genetic variability among the cultivars and within individual cultivars; the difference found can be explained by differences in nutritional demands, which vary within the same species (Marschner, 2012).
The cultivar ‘Shelly’ had higher Zn contents than did ‘Agam’, and ‘Omer’ was statistically equal to both. According to the classification by Quaggio (1996), the cultivar ‘Agam’ had Zn levels within the range considered suitable (between 30 and 50 mg·kg-1), while the cultivars ‘Omer’ and ‘Shelly’ had contents above the range considered adequate (> 50 mg·kg-1). Concerning cultivar development, according to Cohen et al. (2016), because the cultivars ‘Tommy Atkins’ and ‘Kent’ are of the same genetic origin, the Zn contents found for the cultivar ‘Shelly’ are within the optimal range, which is between 51.7 to 168.1 mg·kg-1 for ‘Tommy Atkins’ and 43.7 to 108.9 mg·kg-1 for ‘Kent’ (Rezende et al., 2022). The cultivar ‘Agam’, which results from open pollination of the cultivar ‘Shelly’, also had values within the optimal range proposed by Rezende et al. (2022).
Regardless of the nutrient studied, one should consider that the optimal ranges according to Quaggio (1996) and Rezende et al. (2022) are defined for plants in production and at a different phenological point (flowering). On the other hand, comparing is justified by the absence of specific ranges for seedling formation.
Overall, the Na leaf contents were superior to those of the micronutrients, compatible only with Mn. The higher Na values promoted by ‘Coquinho’ indicate that this rootstock is less selective for the retention of this element and less tolerant when compared to the cultivar ‘Espada’. Mango rootstocks more tolerant to saline stress limit the absorption of toxic ions and their movement to more susceptible organs, controlling influx through the roots (Mahouachi, 2018). When evaluating the effects of ‘Gomera-1’ and ‘Gomera-3’ mango rootstocks on the salinity tolerance of ‘Osteen’ scions, Duran-Zuazo et al. (2004) concluded that the former increased scion tolerance to salt stress, which may be linked to its ability to restrict Cl and Na absorption and transport from roots to shoots. Likewise, Sharma et al. (2016) verified the use of rootstocks in grapefruit (Citrus paradisi Macf.) and verified that the cultivars ‘Limão Ásperos’, ‘Khatti’, and ‘Karna Khatta’ accumulated Na, whereas ‘Laranja-azeda’ excreted it.
The different rootstocks had different nutrient absorption, translocation, and redistribution abilities due to the morphological and physiological aspects of their roots, such as the xylem size (Tombesi et al., 2014; Sarkhosh et al., 2021), affecting nutrient translocation differently in each case. However, as observed, the cultivar used as rootstock with the Israeli mango scions also influences the specific requirements for each nutrient (Lazare et al., 2020).
Deviation from the Optimum Percentage
The negative DOP indexes (DOP < 0) for N, K, and Ca (Table 5) may indicate problems with their availability in the substrate and the absorption by seedlings (Sarkhosh et al., 2021). Concentrations of these nutrients were lower for all rootstock/scion combinations. Notably, very high DOP indexes (DOP > 0) for Mg induce antagonism with other nutrients, such as K and Ca (Yan and Hou, 2018).
The differences in DOP values for macronutrients among rootstock/scion combinations corroborate the findings of Sarkhosh et al. (2021) for Australian commercial cultivars on different rootstocks and those of Yahmed et al. (2022) for different rootstocks and scions of almond trees (Prunus dulcis).
Principal Component Analysis
The principal component analysis (Fig. 5) results demonstrated the importance of selecting proper rootstock/scion combinations to ensure the quality of Israeli mango seedlings. Our findings showed that this factor varied mainly between different genetic materials, with ‘Espada’ being more prone to produce more vigorous seedlings given its greater dry matter accumulation when combined with ‘Agam’ and ‘Omer’. According to some authors, the responses of mango cultivars (rootstock and scion) may be inconsistent, showing great variability in terms of dry matter accumulation and the leaf mineral composition (Sarkhosh et al., 2021; Yahmed et al., 2022). These authors also pointed out that the nutrient balance and association between minerals are important factors to consider when analyzing the nutritional status of mango seedlings.
Conclusions
Dry matter accumulation in Israeli mango seedlings depends on the rootstock selected, with the cultivar ‘Espada’ promoting greater dry matter values, mainly roots and leaves. Nutrient absorption and translocation in Israeli mango seedlings are not only influenced by the rootstock but also by the rootstock/scion combination. According to our results, the cultivar ‘Espada’ should be used as the rootstock for the cultivars ‘Omer’ and ‘Agam’, and ‘Coquinho’ as the rootstock for the cultivar ‘Shelly’. According to the DOP indexes obtained, the Israeli mango cultivar ‘Omer’ shows the greatest nutritional imbalance, demonstrating less compatibility with the evaluated rootstocks. The rootstock/scion combination should be considered when selecting seedlings of Israeli mango cultivars, because when knowing the characteristics of each one, a more targeted and assertive nutritional management scheme can be developed. Based on the results obtained here, it is suggested that future research be directed towards understanding the anatomical, physiological and biochemical interactions in the grafting zone involved in the transport of nutrients from the rootstock to the scions of ‘Agam’, ‘Omer’ and ‘Shelly’ and, based on this, to develop recommendations for adequate nutritional management based on the nutritional status of the plants for the semi-arid conditions of the São Francisco Valley.







